Light-Controlled Destruction and Assembly: Switching between Two Differently Composed Cage-Type Complexes
- PMID: 36215411
- PMCID: PMC10099457
- DOI: 10.1002/anie.202212571
Light-Controlled Destruction and Assembly: Switching between Two Differently Composed Cage-Type Complexes
Abstract
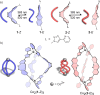
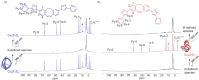
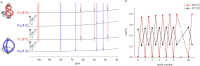
We report on two regioisomeric, diazocine ligands 1 and 2 that can both be photoswitched between the E- and Z-configurations with violet and green light. The self-assembly of the four species (1-Z, 1-E, 2-Z, 2-E) with CoII ions was investigated upon changing the coordination vectors as a function of the ligand configuration (E vs Z) and regioisomer (1 vs 2). With 1-Z, Co2 (1-Z)3 was self-assembled, while a mixture of ill-defined species (oligomers) was observed with 2-Z. Upon photoswitching with 385 nm to the E configurations, the opposite was observed with 1-E forming oligomers and 2-E forming Co2 (2-E)3 . Light-controlled dis/assembly was demonstrated in a ligand competition experiment with sub-stoichiometric amounts of CoII ions; alternating irradiation with violet and green light resulted in the reversible transformation between Co2 (1-Z)3 and Co2 (2-E)3 over multiple cycles without significant fatigue by photoswitching.
Keywords: Diazocine; Isomerization; Photochemistry; Self-Assembly; Supramolecular Chemistry.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

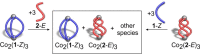
References
-
- Wezenberg S. J., Chem. Lett. 2020, 49, 609–615.
-
- None
-
- Manna D., Udayabhaskararao T., Zhao H., Klajn R., Angew. Chem. Int. Ed. 2015, 54, 12394–12397; - PubMed
- Angew. Chem. 2015, 127, 12571–12574.
Grants and funding
LinkOut - more resources
Full Text Sources

