Connecting sequence features within the disordered C-terminal linker of Bacillus subtilis FtsZ to functions and bacterial cell division
- PMID: 36215496
- PMCID: PMC9586301
- DOI: 10.1073/pnas.2211178119
Connecting sequence features within the disordered C-terminal linker of Bacillus subtilis FtsZ to functions and bacterial cell division
Abstract
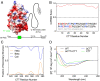
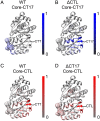
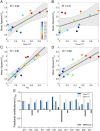
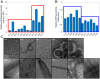
Intrinsically disordered regions (IDRs) can function as autoregulators of folded enzymes to which they are tethered. One example is the bacterial cell division protein FtsZ. This includes a folded core and a C-terminal tail (CTT) that encompasses a poorly conserved, disordered C-terminal linker (CTL) and a well-conserved 17-residue C-terminal peptide (CT17). Sites for GTPase activity of FtsZs are formed at the interface between GTP binding sites and T7 loops on cores of adjacent subunits within dimers. Here, we explore the basis of autoregulatory functions of the CTT in Bacillus subtilis FtsZ (Bs-FtsZ). Molecular simulations show that the CT17 of Bs-FtsZ makes statistically significant CTL-mediated contacts with the T7 loop. Statistical coupling analysis of more than 1,000 sequences from FtsZ orthologs reveals clear covariation of the T7 loop and the CT17 with most of the core domain, whereas the CTL is under independent selection. Despite this, we discover the conservation of nonrandom sequence patterns within CTLs across orthologs. To test how the nonrandom patterns of CTLs mediate CTT-core interactions and modulate FtsZ functionalities, we designed Bs-FtsZ variants by altering the patterning of oppositely charged residues within the CTL. Such alterations disrupt the core-CTT interactions, lead to anomalous assembly and inefficient GTP hydrolysis in vitro and protein degradation, aberrant assembly, and disruption of cell division in vivo. Our findings suggest that viable CTLs in FtsZs are likely to be IDRs that encompass nonrandom, functionally relevant sequence patterns that also preserve three-way covariation of the CT17, the T7 loop, and core domain.
Keywords: autoinhibition; autoregulation; covariation; intrinsically disordered proteins; polymerization.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Brodsky S., Jana T., Barkai N., Order through disorder: The role of intrinsically disordered regions in transcription factor binding specificity. Curr. Opin. Struct. Biol. 71, 110–115 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

