Validation and application of a quantitative LC-MS/MS assay for the analysis of first-line anti-tuberculosis drugs, rifabutin and their metabolites in human breast milk
- PMID: 36215877
- PMCID: PMC9652742
- DOI: 10.1016/j.jchromb.2022.123489
Validation and application of a quantitative LC-MS/MS assay for the analysis of first-line anti-tuberculosis drugs, rifabutin and their metabolites in human breast milk
Abstract
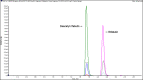
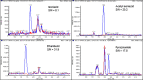
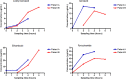
Breast milk is the preferred method of infant nutrition. Breastfeeding infants born to mothers treated for TB may be at risk of drug toxicity through breast milk exposure, or potentially be vulnerable to select for drug resistance with low level drug exposure. Except for isoniazid, the quantification of first-line TB drugs including rifabutin in breast milk has not been previously described and will provide much-needed insight to TB drug exposure in breastfeeding infants. We developed and validated a novel method to quantify several first-line TB drugs and their major metabolites in breast milk. Accuracy and precision were assessed during three consecutive, independent validation batches over a calibration range of 0.300-30.0 µg/mL for isoniazid and ethambutol, 0.150-15.0 µg/mL for acetyl isoniazid, desacetyl rifampicin, rifampicin, and pyrazinamide, 0.0150-1.50 µg/mL for rifabutin, and 0.00751-0.751 µg/mL for deacetyl rifabutin in breast milk. The method was reproducible for all analytes when using breast milk from six different sources and was not influenced by matrix effects with a mean regression precision (CV(%)) ranging between 1.0 and 2.8. The average recovery of analytes from the matrix was 76.7-99.1%, with a CV(%) between 0.4 and 4.4, while the average process efficiency was between 74.4 and 93.1% with a CV(%) between 1.9 and 8.3. Although only acetyl isoniazid, isoniazid, ethambutol, and pyrazinamide were successfully assayed in breast milk, samples taken from mothers treated for rifampicin-resistant TB and the inclusion of all first-line TB drugs, including rifabutin in the assay development and validation process will allow future quantification of these analytes in breast milk.
Keywords: Breast milk; First-line TB drugs; Infant exposure; Rifabutin; Solid phase extraction; Tandem mass spectrometry.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Rapid and highly sensitive quantification of the anti-tuberculosis agents isoniazid, ethambutol, pyrazinamide, rifampicin and rifabutin in human plasma by UPLC-MS/MS.J Pharm Biomed Anal. 2020 Feb 20;180:113076. doi: 10.1016/j.jpba.2019.113076. Epub 2019 Dec 26. J Pharm Biomed Anal. 2020. PMID: 31896523
-
A battery of tandem mass spectrometry assays with stable isotope-dilution for the quantification of 15 anti-tuberculosis drugs and two metabolites in patients with susceptible-, multidrug-resistant- and extensively drug-resistant tuberculosis.J Chromatogr B Analyt Technol Biomed Life Sci. 2022 Nov 15;1211:123456. doi: 10.1016/j.jchromb.2022.123456. Epub 2022 Sep 20. J Chromatogr B Analyt Technol Biomed Life Sci. 2022. PMID: 36240540
-
Simultaneous quantification of isoniazid, rifampicin, ethambutol and pyrazinamide by liquid chromatography/tandem mass spectrometry.APMIS. 2016 Nov;124(11):1004-1015. doi: 10.1111/apm.12590. Epub 2016 Aug 22. APMIS. 2016. PMID: 27546025
-
Optimizing treatment outcome of first-line anti-tuberculosis drugs: the role of therapeutic drug monitoring.Eur J Clin Pharmacol. 2016 Aug;72(8):905-16. doi: 10.1007/s00228-016-2083-4. Epub 2016 Jun 15. Eur J Clin Pharmacol. 2016. PMID: 27305904 Review.
-
Tuberculosis in neonates and infants: epidemiology, pathogenesis, clinical manifestations, diagnosis, and management issues.Paediatr Drugs. 2005;7(4):219-34. doi: 10.2165/00148581-200507040-00002. Paediatr Drugs. 2005. PMID: 16117559 Review.
Cited by
-
Push forward LC-MS-based therapeutic drug monitoring and pharmacometabolomics for anti-tuberculosis precision dosing and comprehensive clinical management.J Pharm Anal. 2024 Jan;14(1):16-38. doi: 10.1016/j.jpha.2023.09.009. Epub 2023 Sep 22. J Pharm Anal. 2024. PMID: 38352944 Free PMC article. Review.
-
Infant Exposure to Antituberculosis Drugs via Breast Milk and Assessment of Potential Adverse Effects in Breastfed Infants: Critical Review of Data.Pharmaceutics. 2023 Apr 13;15(4):1228. doi: 10.3390/pharmaceutics15041228. Pharmaceutics. 2023. PMID: 37111713 Free PMC article. Review.
-
Quantitative Analysis of Isoniazid and Its Four Primary Metabolites in Plasma of Tuberculosis Patients Using LC-MS/MS.Molecules. 2022 Dec 6;27(23):8607. doi: 10.3390/molecules27238607. Molecules. 2022. PMID: 36500699 Free PMC article.
-
Best practices for the care of pregnant people living with TB.Int J Tuberc Lung Dis. 2023 May 1;27(5):357-366. doi: 10.5588/ijtld.23.0031. Int J Tuberc Lung Dis. 2023. PMID: 37143222 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention, “Treatment for TB Disease & Pregnancy,” Centers for Disease Control and Prevention, Aug. 13, 2020. https://www.cdc.gov/tb/topic/treatment/pregnancy.htm (accessed Jun. 21, 2022).
-
- Garessus E.D.G., Mielke H., Gundert-Remy U. Exposure of Infants to Isoniazid via Breast Milk After Maternal Drug Intake of Recommended Doses Is Clinically Insignificant Irrespective of Metaboliser Status. A Physiologically-Based Pharmacokinetic (PBPK) Modelling Approach to Estimate Drug Exposure of Infants via Breast-Feeding. Front. Pharmacol. Jan. 2019;10 doi: 10.3389/fphar.2019.00005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

