Effectiveness and durability of BNT162b2 vaccine against hospital and emergency department admissions due to SARS-CoV-2 omicron sub-lineages BA.1 and BA.2 in a large health system in the USA: a test-negative, case-control study
- PMID: 36216013
- PMCID: PMC9765328
- DOI: 10.1016/S2213-2600(22)00354-X
Effectiveness and durability of BNT162b2 vaccine against hospital and emergency department admissions due to SARS-CoV-2 omicron sub-lineages BA.1 and BA.2 in a large health system in the USA: a test-negative, case-control study
Abstract
Background: The SARS-CoV-2 omicron (B.1.1.529 BA.1) lineage was first detected in November, 2021, and is associated with reduced vaccine effectiveness. By March, 2022, BA.1 had been replaced by sub-lineage BA.2 in the USA. As new variants evolve, vaccine performance must be continually assessed. We aimed to evaluate the effectiveness and durability of BNT162b2 (Pfizer-BioNTech) against hospital and emergency department admissions for BA.1 and BA.2.
Methods: In this test-negative, case-control study, we sourced data from the electronic health records of adult (aged ≥18 years) members of Kaiser Permanente Southern California (KPSC), which is a health-care system in the USA, who were admitted to one of 15 KPSC hospitals or emergency departments (without subsequent hospitalisation) between Dec 27, 2021, and June 4, 2022, with an acute respiratory infection and were tested for SARS-CoV-2 by RT-PCR. Omicron sub-lineage was determined by use of sequencing, spike gene target failure, and the predominance of variants in certain time periods. Our main outcome was the effectiveness of two or three doses of BNT162b2 in preventing emergency department or hospital admission. Variant-specific vaccine effectiveness was evaluated by comparing the odds ratios from logistic regression models of vaccination between test-positive cases and test-negative controls, adjusting for the month of admission, age, sex, race and ethnicity, body-mass index, Charlson Comorbidity Index, previous influenza or pneumococcal vaccines, and previous SARS-CoV-2 infection. We also assessed effectiveness by the time since vaccination. This study is registered at ClinicalTrials.gov, NCT04848584, and is ongoing.
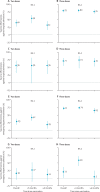
Findings: Of 65 813 total admissions during the study period, we included 16 994 in our analyses, of which 7435 were due to BA.1, 1056 were due to BA.2, and 8503 were not due to SARS-CoV-2. In adjusted analyses, two-dose vaccine effectiveness was 40% (95% CI 27 to 50) for hospitalisation and 29% (18 to 38) for emergency department admission against BA.1 and 56% (31 to 72) for hospitalisation and 16% (-5 to 33) for emergency department admission against BA.2. Three-dose vaccine effectiveness was 79% (74 to 83) for hospitalisation and 72% (67 to 77) for emergency department admission against BA.1 and 71% (55 to 81) for hospitalisation and 21% (1 to 37) for emergency department admission against BA.2. Less than 3 months after the third dose, vaccine effectiveness was 80% (74 to 84) for hospitalisation and 74% (69 to 78) for emergency department admission against BA.1. Vaccine effectiveness 3 months or more after the third dose was 76% (69 to 82) against BA.1-related hospitalisation and 65% (56 to 73) against BA.1-related emergency department admission. Against BA.2, vaccine effectiveness was 74% (47 to 87) for hospitalisation and 59% (40 to 72) for emergency department admission at less than 3 months after the third dose and 70% (53 to 81) for hospitalisation and 5% (-21 to 25) for emergency department admission at 3 months or more after the third dose.
Interpretation: Two doses of BNT162b2 provided only partial protection against BA.1-related and BA.2-related hospital and emergency department admission, which underscores the need for booster doses against omicron. Although three doses offered high levels of protection (≥70%) against hospitalisation, variant-adapted vaccines are probably needed to improve protection against less severe endpoints, like emergency department admission, especially for BA.2.
Funding: Pfizer.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests SRV, LJ, LP, and JMM are employees of, and hold stock, stock options, or both in, Pfizer. SYT, TBF, JMS, VH, and BKA received research support from Pfizer during the conduct of this study, which was paid directly to KPSC. BKA received research support for work unrelated to this study from Pfizer, Moderna, Dynavax, Seqirus, and GlaxoSmithKline. JMS received research support from ALK, Dynavax, and Novavax for work unrelated to this study. TBF previously owned stock in Pfizer. SYT received research support from Genentech and funds from the Centers for Disease Control and Prevention for work unrelated to this study. LP owns stock in Merck & Co. FX declares no competing interests.
Figures
Comment in
-
The elusive goal of COVID-19 vaccine immunity.Lancet Respir Med. 2023 Feb;11(2):115-117. doi: 10.1016/S2213-2600(22)00394-0. Epub 2022 Oct 7. Lancet Respir Med. 2023. PMID: 36216010 Free PMC article. No abstract available.
References
-
- WHO Weekly epidemiological update on COVID-19—25 May 2022. Edition 93. May 25, 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

