Intensive endoscopic resection for downstaging of polyp burden in patients with familial adenomatous polyposis (J-FAPP Study III): a multicenter prospective interventional study
- PMID: 36216266
- PMCID: PMC10060053
- DOI: 10.1055/a-1945-9120
Intensive endoscopic resection for downstaging of polyp burden in patients with familial adenomatous polyposis (J-FAPP Study III): a multicenter prospective interventional study
Abstract
Background: Total colectomy is the standard treatment for familial adenomatous polyposis (FAP). Recently, an increasing number of young patients with FAP have requested the postponement of surgery or have refused to undergo surgery. We aimed to evaluate the effectiveness of intensive endoscopic removal for downstaging of polyp burden (IDP) in FAP.
Method: A single-arm intervention study was conducted at 22 facilities. Participants were patients with FAP, aged ≥ 16 years, who had not undergone colectomy or who had undergone colectomy but had ≥ 10 cm of large intestine remaining. For IDP, colorectal polyps of ≥ 10 mm were removed, followed by polyps of ≥ 5 mm. The primary end point was the presence/absence of colectomy during a 5-year intervention period.
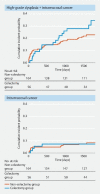
Results: 222 patients were eligible, of whom 166 had not undergone colectomy, 46 had undergone subtotal colectomy with ileorectal anastomosis, and 10 had undergone partial resection of the large intestine. During the intervention period, five patients (2.3 %, 95 % confidence interval [CI] 0.74 %-5.18 %) underwent colectomy, and three patients died. Completion of the 5-year intervention period without colectomy was confirmed in 150 /166 patients who had not undergone colectomy (90.4 %, 95 %CI 84.8 %-94.4 %) and in 47 /56 patients who had previously undergone colectomy (83.9 %, 95 %CI 71.7 %-92.4 %).
Conclusion: IDP in patients with mild-to-moderate FAP could have the potential to be a useful means of preventing colorectal cancer without implementing colectomy. However, if the IDP protocol was proposed during a much longer term, it may not preclude the possibility that a large proportion of colectomies may still need to be performed.
Trial registration: ClinicalTrials.gov NCT03567863.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
T. Takayama received a research grant from Fujifilm Co., Ltd., outside the submitted work. S. Hori received personal fees from Fujifilm Co., Ltd., Fujifilm Medical Co., Ltd., and Kaneka Medix Co., Ltd., outside the submitted work. Y. Takeuchi received personal fees from Olympus, Boston Scientific Japan, Daiichi-Sankyo, Miyarisan Pharmaceutical, Asuka Pharmaceutical, AstraZeneca, EA Pharma, Zeria Pharmaceutical, Fujifilm, Kaneka Medix, and Kyorin Pharmaceutical, outside the submitted work. H. Ishikawa, M. Yamada, Y. Sato, S. Tanaka, C. Akiko, M. Tajika, H. Doyama, Y. Ohda, T. Horimatsu, Y. Sano, K. Tanakaya, H. Ikematsu, Y. Saida, H. Ishida, H. Kashida, S. Kiriyama, K. Lee, J. Tashiro, N. Kobayashi, T. Nakajima, S. Suzuki, and M. Mutoh declare that they have no conflict of interest.
Figures


Comment in
-
Is there a role for endoscopic management of the large bowel in familial adenomatous polyposis?Endoscopy. 2023 Apr;55(4):353-354. doi: 10.1055/a-1990-1046. Epub 2023 Jan 26. Endoscopy. 2023. PMID: 36702130 No abstract available.
References
-
- Iwama T, Tamura K, Morita T et al.A clinical overview of familial adenomatous polyposis derived from the database of the polyposis registry of Japan. Int J Clin Oncol. 2004;9:308–316. - PubMed
-
- Vasen H F, Möslein G, Alonso A et al.Guidelines for the clinical management of familial adenomatous polyposis (FAP) Gut. 2008;57:704–713. - PubMed
-
- Smith A J, Lewis J J, Merchant N B et al.Surgical management of intra-abdominal desmoid tumours. Br J Surg. 2000;87:608–613. - PubMed
-
- Nielsen M, Hes F J, Nagengast F M et al.Germline mutations in APC and MUTYH are responsible for the majority of families with attenuated familial adenomatous polyposis. Clin Genet. 2007;71:427–433. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

