PtOx Cly (OH)z (H2 O)n Complexes under Oxidative and Reductive Conditions: Impact of the Level of Theory on Thermodynamic Stabilities
- PMID: 36216780
- PMCID: PMC10100086
- DOI: 10.1002/cphc.202200711
PtOx Cly (OH)z (H2 O)n Complexes under Oxidative and Reductive Conditions: Impact of the Level of Theory on Thermodynamic Stabilities
Abstract
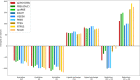
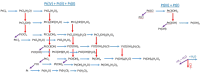
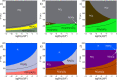
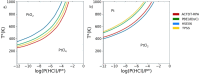
Platinum-based catalysts with Cl- , OH- , O2- and H2 O ligands, are involved in many industrial processes. Their final chemical properties are impacted by calcination and reduction applied during the preparation and activation steps. We investigate their stability under these reactive conditions with density functional theory (DFT). We benchmark various functionals (PBE-dDsC, optPBE, B3LYP, HSE06, PBE0, TPSS, RTPSS and SCAN) against ACFDT-RPA. PBE-dDsC is well adapted, although hybrid functionals are more accurate for redox reactions. Thermodynamic phase diagrams are determined by computing the chemical potential of the species as a function of temperature and partial pressures of H2 O, HCl, O2 and H2 . The stability and nature of the Pt species are highly sensitive to the activation conditions. Under O2 , high temperatures favour PtO2 while under H2 , platinum is easily reduced to Pt(0). Chlorine modifies the coordination sphere of platinum during calcination by stabilizing PtCl4 and shifts the reduction of platinum to higher temperatures under H2 .
Keywords: calcination and reduction conditions; density functional calculations; platinum complexes; random phase approximation; thermodynamic phase diagrams.
© 2022 The Authors. ChemPhysChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zeise W. C., Ann. Phys. Chem. 1831, 97, 497–541.
-
- Clarke M. L., Polyhedron 2001, 20, 151–164.
-
- None
-
- Ertl G., Knözinger H., Weitkamp J., Handbook of heterogeneous catalysis, Wiley-VCH, Weinheim (Germany) 1997;
-
- Chen A., Holt-Hindle P., Chem. Rev. 2010, 110, 3767–3804; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

