Hyperthermia promotes degradation of the acute promyelocytic leukemia driver oncoprotein ZBTB16/RARα
- PMID: 36216898
- PMCID: PMC10042863
- DOI: 10.1038/s41401-022-01001-6
Hyperthermia promotes degradation of the acute promyelocytic leukemia driver oncoprotein ZBTB16/RARα
Abstract
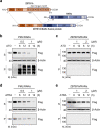
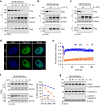
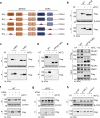
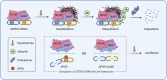
The acute promyelocytic leukemia (APL) driver ZBTB16/RARα is generated by the t(11;17) (q23;q21) chromosomal translocation, which is resistant to combined treatment of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) or conventional chemotherapy, resulting in extremely low survival rates. In the current study, we investigated the effects of hyperthermia on the oncogenic fusion ZBTB16/RARα protein to explore a potential therapeutic approach for this variant APL. We showed that Z/R fusion protein expressed in HeLa cells was resistant to ATO, ATRA, and conventional chemotherapeutic agents. However, mild hyperthermia (42 °C) rapidly destabilized the ZBTB16/RARα fusion protein expressed in HeLa, 293T, and OCI-AML3 cells, followed by robust ubiquitination and proteasomal degradation. In contrast, hyperthermia did not affect the normal (i.e., unfused) ZBTB16 and RARα proteins, suggesting a specific thermal sensitivity of the ZBTB16/RARα fusion protein. Importantly, we found that the destabilization of ZBTB16/RARα was the initial step for oncogenic fusion protein degradation by hyperthermia, which could be blocked by deletion of nuclear receptor corepressor (NCoR) binding sites or knockdown of NCoRs. Furthermore, SIAH2 was identified as the E3 ligase participating in hyperthermia-induced ubiquitination of ZBTB16/RARα. In short, these results demonstrate that hyperthermia could effectively destabilize and subsequently degrade the ZBTB16/RARα fusion protein in an NCoR-dependent manner, suggesting a thermal-based therapeutic strategy that may improve the outcome in refractory ZBTB16/RARα-driven APL patients in the clinic.
Keywords: ZBTB16/RARα fusion protein; acute promyelocytic leukemia; arsenicals; hyperthermia; nuclear receptor corepressors; proteolysis.
© 2022. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Targeting HDAC3 to overcome the resistance to ATRA or arsenic in acute promyelocytic leukemia through ubiquitination and degradation of PML-RARα.Cell Death Differ. 2023 May;30(5):1320-1333. doi: 10.1038/s41418-023-01139-8. Epub 2023 Mar 9. Cell Death Differ. 2023. PMID: 36894687 Free PMC article.
-
ZBTB16-RARα-Positive Atypical Promyelocytic Leukemia: A Case Report.Medicina (Kaunas). 2022 Apr 6;58(4):520. doi: 10.3390/medicina58040520. Medicina (Kaunas). 2022. PMID: 35454359 Free PMC article.
-
Hyperthermia Selectively Destabilizes Oncogenic Fusion Proteins.Blood Cancer Discov. 2021 May 11;2(4):388-401. doi: 10.1158/2643-3230.BCD-20-0188. eCollection 2021 Jul. Blood Cancer Discov. 2021. PMID: 34661159 Free PMC article.
-
Acute promyelocytic leukemia and variant fusion proteins: PLZF-RARα fusion protein at a glance.Semin Oncol. 2019 Apr;46(2):133-144. doi: 10.1053/j.seminoncol.2019.04.004. Epub 2019 May 7. Semin Oncol. 2019. PMID: 31126665 Review.
-
[Acute promyelocytic leukemia: state-of-the-art management].Rinsho Ketsueki. 2018;59(6):725-734. doi: 10.11406/rinketsu.59.725. Rinsho Ketsueki. 2018. PMID: 29973452 Review. Japanese.
Cited by
-
Identification of E3 ubiquitin ligase-associated prognostic genes and construction of a prediction model for uterine cervical cancer based on bioinformatics analysis.Discov Oncol. 2024 Aug 31;15(1):395. doi: 10.1007/s12672-024-01271-y. Discov Oncol. 2024. PMID: 39217222 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

