The Burden of Proof studies: assessing the evidence of risk
- PMID: 36216935
- PMCID: PMC9556298
- DOI: 10.1038/s41591-022-01973-2
The Burden of Proof studies: assessing the evidence of risk
Abstract
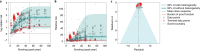
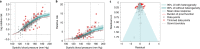
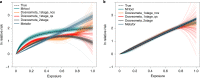
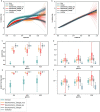
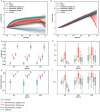
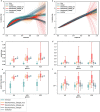
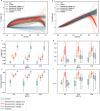
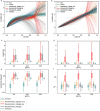
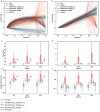
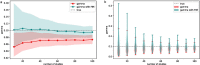
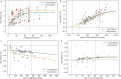
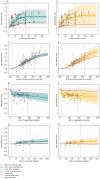
Exposure to risks throughout life results in a wide variety of outcomes. Objectively judging the relative impact of these risks on personal and population health is fundamental to individual survival and societal prosperity. Existing mechanisms to quantify and rank the magnitude of these myriad effects and the uncertainty in their estimation are largely subjective, leaving room for interpretation that can fuel academic controversy and add to confusion when communicating risk. We present a new suite of meta-analyses-termed the Burden of Proof studies-designed specifically to help evaluate these methodological issues objectively and quantitatively. Through this data-driven approach that complements existing systems, including GRADE and Cochrane Reviews, we aim to aggregate evidence across multiple studies and enable a quantitative comparison of risk-outcome pairs. We introduce the burden of proof risk function (BPRF), which estimates the level of risk closest to the null hypothesis that is consistent with available data. Here we illustrate the BPRF methodology for the evaluation of four exemplar risk-outcome pairs: smoking and lung cancer, systolic blood pressure and ischemic heart disease, vegetable consumption and ischemic heart disease, and unprocessed red meat consumption and ischemic heart disease. The strength of evidence for each relationship is assessed by computing and summarizing the BPRF, and then translating the summary to a simple star rating. The Burden of Proof methodology provides a consistent way to understand, evaluate and summarize evidence of risk across different risk-outcome pairs, and informs risk analysis conducted as part of the Global Burden of Diseases, Injuries, and Risk Factors Study.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

