Improving outcomes for patients with lymphoma: design and development of the Australian and New Zealand Lymphoma and Related Diseases Registry
- PMID: 36217114
- PMCID: PMC9549605
- DOI: 10.1186/s12874-022-01728-0
Improving outcomes for patients with lymphoma: design and development of the Australian and New Zealand Lymphoma and Related Diseases Registry
Abstract
Background: Lymphoma is a malignancy of lymphocytes and lymphoid tissues comprising a heterogeneous group of diseases, with up to 80 entities now described. Lymphoma is the 6th most common cancer in Australia, affecting patients of all ages, with rising incidence rates. With the proliferation of efficacious novel agents, therapeutic strategies are increasingly diverse and survival is improving. There is a clear need for contemporary robust and detailed data on diagnostic, investigational and management strategies for this disease in Australia, New Zealand and worldwide, to inform and benchmark local and international standards of care. Clinical quality registries can provide these data, and support development of strategies to address variations in management, including serving as platforms for clinical trials and other research activities. The Lymphoma and Related Diseases Registry (LaRDR) was developed to capture details of patient demographics, disease characteristics, and management throughout their disease course and therapy and to develop outcome benchmarks nationally and internationally for lymphoma. This report describes the aims, development and implementation of the LaRDR, as well as challenges addressed in the process.
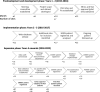
Methods: The LaRDR was established in 2016 as a multicentre, collaborative project at sites across Australia with a secure online database which collects prospective data on patients with a new diagnosis of lymphoma or chronic lymphocytic leukaemia (CLL). LaRDR development required multidisciplinary participation including specialist haematology, information technology, and biostatistical support, as well as secure funding. Here we describe the database development, data entry, ethics approval process, registry governance and support for participating sites and the coordinating centre.
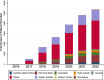
Results: To date more than 5,300 patients have been enrolled from 28 sites in Australia and New Zealand. Multiple challenges arose during the development, which we describe, along with approaches used to overcome them. Several confirmed international collaborations are now in place, and the registry is providing valuable data for clinicians, researchers, industry and government, including through presentations of results at major national and international conferences.
Conclusion: Challenges in establishing the LaRDR have been successfully overcome and the registry is now a valuable resource for lymphoma clinicians, researchers, health economists and others in Australia, New Zealand and globally.
Keywords: Chronic lymphocytic leukaemia; Clinical quality registry; Epidemiology; Lymphoma.
© 2022. The Author(s).
Conflict of interest statement
None reported
Figures
References
-
- Commonwealth of Australia . Strategic Review of Health and Medical Research in Australia – Better health through research. 2013.
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edition, Volume 2. Lyon: IARC; 2017.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical