High platelet-to-lymphocyte ratios in triple-negative breast cancer associates with immunosuppressive status of TILs
- PMID: 36217150
- PMCID: PMC9552414
- DOI: 10.1186/s13058-022-01563-7
High platelet-to-lymphocyte ratios in triple-negative breast cancer associates with immunosuppressive status of TILs
Abstract
Background: Rating lymphocytes (TILs) are a prognostic marker in breast cancer and high TIL infiltration correlates with better patient outcomes. Meanwhile, parameters involving immune cells in peripheral blood have also been established as prognostic markers. High platelet-to-lymphocyte ratios (PLRs) and neutrophil-to-lymphocyte ratios (NLRs) are related to poor outcomes in breast cancer, but their mechanisms remain unknown. To date, TILs and these parameters have been examined separately.
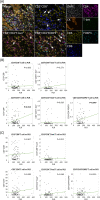
Methods: We investigated the relationship between TILs and the peripheral blood markers, PLR and NLR, in the same patients, using surgical specimens from 502 patients with invasive breast carcinoma without preoperative chemotherapy. For analysis of triple-negative breast cancer (TNBC) patient outcomes, 59 patients who received preoperative chemotherapy were also examined. For immune cell profiling, multiplexed fluorescent immunohistochemistry (mfIHC) of CD3, CD4, CD8, FOXP3 and T-bet, was conducted.
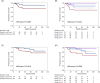
Results: A positive correlation between PLR and TIL was observed in TNBC (P = 0.013). On mfIHC, tumors in patients with high PLR and NLR contained more CD3+CD4+FOXP3+ T-cells (P = 0.049 and 0.019, respectively), while no trend was observed in CD8+ T-cells. TNBC patients had different patterns of outcomes according to TIL and PLR, with the TIL-high/PLR-low group having the lowest rate of disease relapse and death, and the longest distant metastasis-free and overall survivals, while the TIL-low/PLR-high group had the shortest survivals.
Conclusions: Our data suggest that the combination of PLR with TIL assessment may enable more accurate prediction of patient outcomes with TNBC.
Keywords: Multiplexed fluorescent immunohistochemistry; Platelet-to-lymphocyte ratio; Regulatory T-cells; Triple-negative breast cancer; Tumor-infiltrating lymphocyte.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study.Breast Cancer Res. 2015 Sep 4;17(1):124. doi: 10.1186/s13058-015-0632-x. Breast Cancer Res. 2015. PMID: 26341640 Free PMC article.
-
Predictive factors for response to neoadjuvant chemotherapy: inflammatory and immune markers in triple-negative breast cancer.Breast Cancer. 2023 Nov;30(6):1085-1093. doi: 10.1007/s12282-023-01504-y. Epub 2023 Oct 2. Breast Cancer. 2023. PMID: 37782377
-
Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis.BMC Cancer. 2020 Mar 4;20(1):179. doi: 10.1186/s12885-020-6668-z. BMC Cancer. 2020. PMID: 32131780 Free PMC article.
-
Tumor infiltrating lymphocytes and neutrophil-to-lymphocyte ratio in relation to pathological complete remission to neoadjuvant therapy and prognosis in triple negative breast cancer.Pathol Res Pract. 2023 Aug;248:154687. doi: 10.1016/j.prp.2023.154687. Epub 2023 Jul 13. Pathol Res Pract. 2023. PMID: 37478522
-
The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis.Breast Cancer Res Treat. 2014 Dec;148(3):467-76. doi: 10.1007/s10549-014-3185-2. Epub 2014 Nov 1. Breast Cancer Res Treat. 2014. PMID: 25361613 Review.
Cited by
-
Real‑world evaluation of the efficacy of immune checkpoint inhibitors in the treatment of metastatic breast cancer.Oncol Lett. 2024 Oct 25;29(1):29. doi: 10.3892/ol.2024.14775. eCollection 2025 Jan. Oncol Lett. 2024. PMID: 39512498 Free PMC article.
-
Circulating blood biomarkers correlated with the prognosis of advanced triple negative breast cancer.BMC Womens Health. 2024 Jan 13;24(1):38. doi: 10.1186/s12905-023-02871-6. BMC Womens Health. 2024. PMID: 38218823 Free PMC article.
-
Dynamics of tumor in situ fluid circulating tumor DNA in recurrent glioblastomas forecasts treatment efficacy of immune checkpoint blockade coupled with low-dose bevacizumab.J Cancer Res Clin Oncol. 2024 Oct 18;150(10):466. doi: 10.1007/s00432-024-05997-8. J Cancer Res Clin Oncol. 2024. PMID: 39422764 Free PMC article. Clinical Trial.
-
Dynamic monocyte changes as prognostic indicators in operable gastric cancer: a retrospective cohort analysis.Front Oncol. 2025 Feb 7;15:1514281. doi: 10.3389/fonc.2025.1514281. eCollection 2025. Front Oncol. 2025. PMID: 39990694 Free PMC article.
-
A real-world study of immune checkpoint inhibitors in advanced triple-negative breast cancer.Cancer Innov. 2023 Apr 23;2(3):172-180. doi: 10.1002/cai2.70. eCollection 2023 Jun. Cancer Innov. 2023. PMID: 38089401 Free PMC article.
References
-
- Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. - DOI - PubMed
-
- Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase iii randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. - DOI - PMC - PubMed
-
- Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im S-A, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–1828. doi: 10.1016/S0140-6736(20)32531-9. - DOI - PubMed
-
- Zou Y, Zou X, Zheng S, Tang H, Zhang L, Liu P, et al. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: a systematic review and meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920940928–1758835920940928. doi: 10.1177/1758835920940928. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

