Applying implementation frameworks to the clinical trial context
- PMID: 36217172
- PMCID: PMC9552519
- DOI: 10.1186/s43058-022-00355-6
Applying implementation frameworks to the clinical trial context
Erratum in
-
Correction: Applying implementation frameworks to the clinical trial context.Implement Sci Commun. 2023 Mar 14;4(1):27. doi: 10.1186/s43058-023-00413-7. Implement Sci Commun. 2023. PMID: 36918958 Free PMC article. No abstract available.
Abstract
Background: Clinical trials advance science, benefit society, and provide optimal care to individuals with some conditions, such as cancer. However, clinical trials often fail to reach their endpoints, and low participant enrollment remains a critical problem with trial conduct. In these ways, clinical trials can be considered beneficial evidence-based practices suffering from poor implementation. Prior approaches to improving trials have had difficulties with reproducibility and limited impact, perhaps due to the lack of an underlying trial improvement framework. For these reasons, we propose adapting implementation science frameworks to the clinical trial context to improve the implementation of clinical trials.
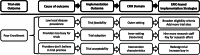
Main text: We adapted an outcomes framework (Proctor's Implementation Outcomes Framework) and a determinants framework (the Consolidated Framework for Implementation Research) to the trial context. We linked these frameworks to ERIC-based improvement strategies and present an inferential process model for identifying and selecting trial improvement strategies based on the Implementation Research Logic Model. We describe example applications of the framework components to the trial context and present a worked example of our model applied to a trial with poor enrollment. We then consider the implications of this approach on improving existing trials, the design of future trials, and assessing trial improvement interventions. Additionally, we consider the use of implementation science in the clinical trial context, and how clinical trials can be "test cases" for implementation research.
Conclusions: Clinical trials can be considered beneficial evidence-based interventions suffering from poor implementation. Adapting implementation science approaches to the clinical trial context can provide frameworks for contextual assessment, outcome measurement, targeted interventions, and a shared vocabulary for clinical trial improvement. Additionally, exploring implementation frameworks in the trial context can advance the science of implementation through both "test cases" and providing fertile ground for implementation intervention design and testing.
Keywords: Clinical trials; Consolidated framework for implementation research; Determinants; Implementation mapping; Implementation outcomes; Implementation research logic model.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that Anne Sales is co-Editor-in-Chief of the journal.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Applying the updated MRC framework for developing and evaluating complex interventions with integrated implementation conceptual knowledge: an example using NeuroRehabilitation OnLine.Front Health Serv. 2025 May 6;5:1562627. doi: 10.3389/frhs.2025.1562627. eCollection 2025. Front Health Serv. 2025. PMID: 40395560 Free PMC article.
-
Implementation Science Using Proctor's Framework and an Adaptation of the Multiphase Optimization Strategy: Optimizing a Financial Incentive Intervention for HIV Treatment Adherence in Tanzania.J Acquir Immune Defic Syndr. 2019 Dec;82 Suppl 3(Suppl 3):S332-S338. doi: 10.1097/QAI.0000000000002196. J Acquir Immune Defic Syndr. 2019. PMID: 31764271 Free PMC article.
-
The Building Blocks of Implementation Frameworks and Models in Primary Care: A Narrative Review.Front Public Health. 2021 Aug 3;9:675171. doi: 10.3389/fpubh.2021.675171. eCollection 2021. Front Public Health. 2021. PMID: 34414155 Free PMC article. Review.
-
Systematic review of the Exploration, Preparation, Implementation, Sustainment (EPIS) framework.Implement Sci. 2019 Jan 5;14(1):1. doi: 10.1186/s13012-018-0842-6. Implement Sci. 2019. PMID: 30611302 Free PMC article.
Cited by
-
Developing the Meaning-Centered Program for Chinese Americans with Advanced Cancer: Applying Cultural Adaptation Frameworks.Asian Am J Psychol. 2024 Sep;15(3):262-273. doi: 10.1037/aap0000318. Asian Am J Psychol. 2024. PMID: 39430038
-
Translating and communicating evidence on allergy prevention in children to parents: implementation study protocol.Arch Public Health. 2025 Feb 14;83(1):36. doi: 10.1186/s13690-025-01534-2. Arch Public Health. 2025. PMID: 39953614 Free PMC article.
-
Implementation research logic model in the design and execution of eHealth innovations for maternal and newborn healthcare in Ethiopia.Health Res Policy Syst. 2025 Jan 6;23(1):4. doi: 10.1186/s12961-024-01259-8. Health Res Policy Syst. 2025. PMID: 39762955 Free PMC article.
-
Neoadjuvant chemotherapy with or without metformin in early-stage or locally advanced (non-metastatic) triple negative breast cancer-an open-label phase 2 randomized controlled trial.Front Oncol. 2025 Jun 25;15:1561692. doi: 10.3389/fonc.2025.1561692. eCollection 2025. Front Oncol. 2025. PMID: 40636683 Free PMC article.
-
Correction: Applying implementation frameworks to the clinical trial context.Implement Sci Commun. 2023 Mar 14;4(1):27. doi: 10.1186/s43058-023-00413-7. Implement Sci Commun. 2023. PMID: 36918958 Free PMC article. No abstract available.
References
-
- Grand View Research. Clinical trials market size, share & trends analysis report by phase (phase I, phase II, phase III, phase IV), by study design (interventional, observational, expanded access), by indication, by region, and segment forecasts, 2021–2028. Available from: https://www.grandviewresearch.com/industry-analysis/global-clinical-tria.... cited 2021 Jul 30
-
- NCCN Guidelines. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. cited 2021 Feb 5
Grants and funding
LinkOut - more resources
Full Text Sources