Predicting the disease severity in male individuals with ornithine transcarbamylase deficiency
- PMID: 36217298
- PMCID: PMC9639638
- DOI: 10.1002/acn3.51668
Predicting the disease severity in male individuals with ornithine transcarbamylase deficiency
Abstract
Objective: Ornithine transcarbamylase deficiency (OTC-D) is an X-linked metabolic disease and the most common urea cycle disorder. Due to high phenotypic heterogeneity, ranging from lethal neonatal hyperammonemic events to moderate symptoms and even asymptomatic individuals, the prediction of the disease course at an early disease stage is very important to individually adjust therapies such as medical treatment or liver transplantation. In this translational study, we developed a severity-adjusted classification system based on in vitro residual enzymatic OTC activity.
Methods: Applying a cell-based expression system, residual enzymatic OTC activities of 71 pathogenic OTC variants were spectrophotometrically determined and subsequently correlated with clinical and biochemical outcome parameters of 119 male individuals with OTC-D (mOTC-D) as reported in the UCDC and E-IMD registries.
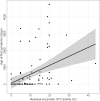
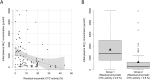
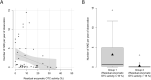
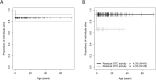
Results: Integration of multiple data sources enabled the establishment of a robust disease prediction model for mOTC-D. Residual enzymatic OTC activity not only correlates with age at first symptoms, initial peak plasma ammonium concentration and frequency of metabolic decompensations but also predicts mortality. The critical threshold of 4.3% residual enzymatic activity distinguishes a severe from an attenuated phenotype.
Interpretation: Residual enzymatic OTC activity reliably predicts the disease severity in mOTC-D and could thus serve as a tool for severity-adjusted evaluation of therapeutic strategies and counselling patients and parents.
© 2022 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
SK received EU funding for the European registry and network for Intoxication type Metabolic Diseases (E‐IMD; CHAFEA agreement no. 2010 12 01). SK receives funding from Immedica Pharma AB for the European Post‐Authorization Registry for Ravicti® (glycerol phenylbutyrate) oral liquid in partnership with the E‐IMD (RRPE) (EU PAS Register no. EUPAS17267;
Figures
References
-
- Bachmann C. Outcome and survival of 88 patients with urea cycle disorders: a retrospective evaluation. Eur J Pediatr. 2003. Jun;162(6):410‐416. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

