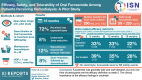
Efficacy, Safety, and Tolerability of Oral Furosemide Among Patients Receiving Hemodialysis: A Pilot Study
- PMID: 36217511
- PMCID: PMC9546731
- DOI: 10.1016/j.ekir.2022.07.003
Efficacy, Safety, and Tolerability of Oral Furosemide Among Patients Receiving Hemodialysis: A Pilot Study
Abstract
Introduction: Diuretic use may reduce volume-related complications in hemodialysis. We evaluated the efficacy, safety, and tolerability of furosemide in patients with hemodialysis-dependent kidney failure.
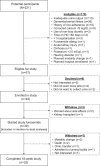
Methods: We conducted an open label, single-arm, 18-week, dose titration pilot study of oral furosemide (maximum dose 320 mg/day) among patients receiving maintenance hemodialysis who reported at least 1 cup of urine output per day. The primary efficacy outcome was an increase from baseline to a specified threshold of 24-hour urine volume, with the threshold based on baseline urine volume (<200 ml/day vs. ≥200 ml/day). Safety outcomes included hypokalemia and hypomagnesemia, and tolerability was assessed by prespecified patient-reported symptoms.
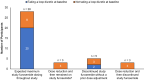
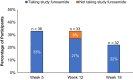
Results: Of the 39 participants, 28 (72%) received the expected furosemide dose, 3 (8%) underwent dose reduction, 5 (12%) discontinued furosemide without dose reduction, and 3 (8%) underwent dose reduction and subsequently discontinued furosemide. The median (quartile 1, quartile 3) baseline 24-hour urine volume was 290 ml (110, 740), and the maximum, average daily study furosemide dose ranged from 69 mg/day to 320 mg/d. The urine output efficacy outcome was met by 12 (33%), 11 (33%), and 7 (22%) participants at weeks 5, 12, and 18, respectively, in the intention-to-treat analysis, and by 12 (39%), 9 (35%), and 7 (28%) participants at weeks 5, 12, and 18, respectively, in the on-treatment analysis. There were no electrolyte, furosemide level, or patient-reported hearing change safety events.
Conclusion: Furosemide was generally safe and well tolerated, but only one-third of participants met the efficacy definition at week 5. The clinical importance of the efficacy findings is uncertain.
Keywords: diuretic; efficacy; furosemide; hemodialysis; hypervolemia; safety.
© 2022 International Society of Nephrology. Published by Elsevier Inc.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

