Lessons learned from pre-clinical testing of xenogeneic decellularized esophagi in a rabbit model
- PMID: 36217545
- PMCID: PMC9547295
- DOI: 10.1016/j.isci.2022.105174
Lessons learned from pre-clinical testing of xenogeneic decellularized esophagi in a rabbit model
Abstract
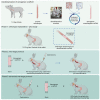
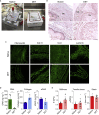
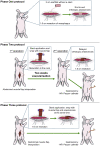
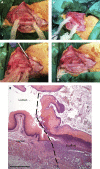
Decellularization of esophagi from several species for tissue engineering is well described, but successful implantation in animal models of esophageal replacement has been challenging. The purpose of this study was to assess feasibility and applicability of esophageal replacement using decellularized porcine esophageal scaffolds in a new pre-clinical model. Following surgical replacement in rabbits with a vascularizing muscle flap, we observed successful anastomoses of decellularized scaffolds, cues of early neovascularization, and prevention of luminal collapse by the use of biodegradable stents. However, despite the success of the surgical procedure, the long-term survival was limited by the fragility of the animal model. Our results indicate that transplantation of a decellularized porcine scaffold is possible and vascular flaps may be useful to provide a vascular supply, but long-term outcomes require further pre-clinical testing in a different large animal model.
Keywords: Biological sciences; Biomedical engineering; Biotechnology; Tissue engineering.
© 2022.
Conflict of interest statement
P.D.C. and L.U. are named inventors of patent application No. PCT/EP2016/071114 and P.D.C. and L.U. are named inventors of UK patent application No. 1708729.7. The remaining authors declare no competing interests.
Figures



References
-
- Catry J., Luong-Nguyen M., Arakelian L., Poghosyan T., Bruneval P., Domet T., Michaud L., Sfeir R., Gottrand F., Cattan P., et al. Circumferential esophageal replacement by a tissue-engineered substitute using mesenchymal stem cells. Cell Transplant. 2017;26:1831–1839. doi: 10.1177/0963689717741498. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

