High-fat diet-induced obesity augments the deleterious effects of estrogen deficiency on bone: Evidence from ovariectomized mice
- PMID: 36217558
- PMCID: PMC9741509
- DOI: 10.1111/acel.13726
High-fat diet-induced obesity augments the deleterious effects of estrogen deficiency on bone: Evidence from ovariectomized mice
Abstract
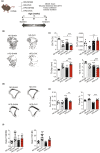
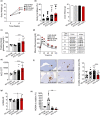
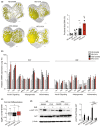
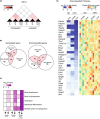
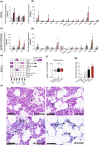
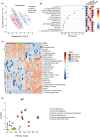
Several epidemiological studies have suggested that obesity complicated with insulin resistance and type 2 diabetes exerts deleterious effects on the skeleton. While obesity coexists with estrogen deficiency in postmenopausal women, their combined effects on the skeleton are poorly studied. Thus, we investigated the impact of high-fat diet (HFD) on bone and metabolism of ovariectomized (OVX) female mice (C57BL/6J). OVX or sham operated mice were fed either HFD (60%fat) or normal diet (10%fat) for 12 weeks. HFD-OVX group exhibited pronounced increase in body weight (~86% in HFD and ~122% in HFD-OVX, p < 0.0005) and impaired glucose tolerance. Bone microCT-scanning revealed a pronounced decrease in trabecular bone volume/total volume (BV/TV) (-15.6 ± 0.48% in HFD and -37.5 ± 0.235% in HFD-OVX, p < 0.005) and expansion of bone marrow adipose tissue (BMAT; +60.7 ± 9.9% in HFD vs. +79.5 ± 5.86% in HFD-OVX, p < 0.005). Mechanistically, HFD-OVX treatment led to upregulation of genes markers of senescence, bone resorption, adipogenesis, inflammation, downregulation of gene markers of bone formation and bone development. Similarly, HFD-OVX treatment resulted in significant changes in bone tissue levels of purine/pyrimidine and Glutamate metabolisms, known to play a regulatory role in bone metabolism. Obesity and estrogen deficiency exert combined deleterious effects on bone resulting in accelerated cellular senescence, expansion of BMAT and impaired bone formation leading to decreased bone mass. Our results suggest that obesity may increase bone fragility in postmenopausal women.
Keywords: Aging, bone fragility; accelerated aging; bone marrow adiposity; menopause; obesity; osteoporosis; senescence.
© 2022 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- Ambrosi, T. H. , Scialdone, A. , Graja, A. , Gohlke, S. , Jank, A. M. , Bocian, C. , Woelk, L. , Fan, H. , Logan, D. W. , Schurmann, A. , Saraiva, L. R. , & Schulz, T. J. (2017). Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell‐based hematopoietic and bone regeneration. Cell Stem Cell, 20, 771–784.e6. - PMC - PubMed
-
- Andreoli, A. , Bazzocchi, A. , Celi, M. , Lauro, D. , Sorge, R. , Tarantino, U. , & Guglielmi, G. (2011). Relationship between body composition, body mass index and bone mineral density in a large population of normal, osteopenic and osteoporotic women. La Radiologia Medica, 116, 1115–1123. - PubMed
-
- Baar, M. P. , Brandt, R. M. C. , Putavet, D. A. , Klein, J. D. D. , Derks, K. W. J. , Bourgeois, B. R. M. , Stryeck, S. , Rijksen, Y. , van Willigenburg, H. , Feijtel, D. A. , van der Pluijm, I. , Essers, J. , van Cappellen, W. A. , van IJcken, W. F. , Houtsmuller, A. B. , Pothof, J. , De Bruin, R. W. F. , Madl, T. , Hoeijmakers, J. H. J. , … De Keizer, P. L. J. (2017). Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell, 169, 132–147.e16. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

