Midterm Durability and Structural Valve Degeneration of Transcatheter Aortic Valve Replacement in a Federal Facility
- PMID: 36217736
- PMCID: PMC9761483
- DOI: 10.1177/15569845221123259
Midterm Durability and Structural Valve Degeneration of Transcatheter Aortic Valve Replacement in a Federal Facility
Abstract
Objective: Transcatheter aortic valve replacement (TAVR), previously reserved for patients of intermediate to prohibitive surgical risk, has now been expanded to patients of any surgical risk with severe aortic stenosis. Bioprostheses are prone to structural valve degeneration (SVD), a progressive and multifactorial process that limits valve durability. As the population undergoing TAVR shifts toward a lower-risk and younger profile, long-term durability is a crucial determinant for patient outcomes. Our objective was to determine the incidence and risk factors of SVD at midterm follow-up in a veteran TAVR population.
Methods: Patients undergoing TAVR at our federal facility were retrospectively evaluated for SVD and other endpoints with standardized consensus criteria. Multivariable Cox proportional hazards analysis was performed to evaluate risk factors for mortality and SVD.
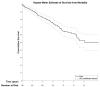
Results: From 2013 to 2020, 344 patients (median age, 78 years) underwent TAVR. Survival from all-cause mortality was 91.3% at 1 year, 75.1% at 3 years, and 61.7% at 5 years. Cumulative freedom from SVD was 98.2% at 1 year, 96.5% at 3 years, and 93.7% at 5 years. All 13 patients with SVD met hemodynamic criteria, and 1 required intervention. Median time to hemodynamic SVD was 1.04 years. Independent risk factors for SVD included age (hazard ratio [HR] = 0.92, 95% confidence interval [CI]: 0.86 to 0.99) and valve size (HR = 0.19, 95% CI: 0.04 to 0.89).
Conclusions: SVD was evident at a low but detectable rate at 5-year follow-up. Further understanding of TAVR biomechanics as well as continued longer-term follow-up will be essential for informing patient-specific risk of SVD.
Keywords: midterm durability; structural valve degeneration; transcatheter aortic valve replacement.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Tseng and Dr. Ge disclose they are founders of ReValve Med, Inc.
Figures


References
-
- Wu C, Vasseur B, Maisel W. The march of transcatheter aortic valve replacement therapy—US Food and Drug Administration perspectives on device approval for patients at low surgical risk. JAMA Cardiol 2020; 5: 5–6. - PubMed
-
- Mack MJ, Leon MB, Thourani VH, et al.. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019; 380: 1695–1705. - PubMed
-
- Popma JJ, Deeb GM, Yakubov SJ, et al.. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019; 380: 1706–1715. - PubMed
-
- Waksman R, Rogers T, Torguson R, et al.. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018; 72: 2095–2105. - PubMed
-
- Sellers SL, Turner CT, Sathananthan J, et al.. Transcatheter aortic heart valves: histological analysis providing insight to leaflet thickening and structural valve degeneration. J Am Coll Cardiol Img 2019; 12: 135–145. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

