Low-molecular weight inhibitors of the alternative complement pathway
- PMID: 36217774
- PMCID: PMC10092480
- DOI: 10.1111/imr.13143
Low-molecular weight inhibitors of the alternative complement pathway
Abstract
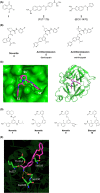
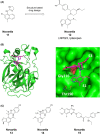
Dysregulation of the alternative complement pathway predisposes individuals to a number of diseases. It can either be evoked by genetic alterations in or by stabilizing antibodies to important pathway components and typically leads to severe diseases such as paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, C3 glomerulopathy, and age-related macular degeneration. In addition, the alternative pathway may also be involved in many other diseases where its amplifying function for all complement pathways might play a role. To identify specific alternative pathway inhibitors that qualify as therapeutics for these diseases, drug discovery efforts have focused on the two central proteases of the pathway, factor B and factor D. Although drug discovery has been challenging for a number of reasons, potent and selective low-molecular weight (LMW) oral inhibitors have now been discovered for both proteases and several molecules are in clinical development for multiple complement-mediated diseases. While the clinical development of these inhibitors initially focuses on diseases with systemic and/or peripheral tissue complement activation, the availability of LMW inhibitors may also open up the prospect of inhibiting complement in the central nervous system where its activation may also play an important role in several neurodegenerative diseases.
Keywords: alternative complement pathway; complement factor B; complement factor D; complement therapeutics; drug discovery; low-molecular weight inhibitor.
© 2022 Novartis Pharma AG. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors are employees of Novartis Pharma AG.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

