Novel multicellular prokaryote discovered next to an underground stream
- PMID: 36217817
- PMCID: PMC9555858
- DOI: 10.7554/eLife.71920
Novel multicellular prokaryote discovered next to an underground stream
Abstract
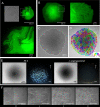
A diversity of prokaryotes currently exhibit multicellularity with different generation mechanisms in a variety of contexts of ecology on Earth. In the present study, we report a new type of multicellular bacterium, HS-3, isolated from an underground stream. HS-3 self-organizes its filamentous cells into a layer-structured colony with the properties of a nematic liquid crystal. After maturation, the colony starts to form a semi-closed sphere accommodating clusters of coccobacillus daughter cells and selectively releases them upon contact with water. This is the first report that shows that a liquid-crystal status of cells can support the prokaryotic multicellular behavior. Importantly, the observed behavior of HS-3 suggests that the recurrent intermittent exposure of colonies to water flow in the cave might have been the ecological context that cultivated the evolutionary transition from unicellular to multicellular life. This is the new extant model that underpins theories regarding a role of ecological context in the emergence of multicellularity.
Keywords: competition-dispersal trade-off; ecological scaffolding; evolution; evolutionary biology; limestone cave; origin of multicellularity; two-dimensional nematic liquid crystal.
© 2022, Mizuno et al.
Conflict of interest statement
KM, MM, TN, AK, SH, SF, KT, KM No competing interests declared
Figures







Comment in
- doi: 10.7554/eLife.83296
Similar articles
-
Illuminating a new path to multicellularity.Elife. 2022 Oct 11;11:e83296. doi: 10.7554/eLife.83296. Elife. 2022. PMID: 36217823 Free PMC article.
-
Loss of Filamentous Multicellularity in Cyanobacteria: the Extremophile Gloeocapsopsis sp. Strain UTEX B3054 Retained Multicellular Features at the Genomic and Behavioral Levels.J Bacteriol. 2020 May 27;202(12):e00514-19. doi: 10.1128/JB.00514-19. Print 2020 May 27. J Bacteriol. 2020. PMID: 32253342 Free PMC article.
-
Evolution of multicellularity by collective integration of spatial information.Elife. 2020 Oct 16;9:e56349. doi: 10.7554/eLife.56349. Elife. 2020. PMID: 33064078 Free PMC article.
-
Selective factors in the evolution of multicellularity in choanoflagellates.J Exp Zool B Mol Dev Evol. 2021 Apr;336(3):315-326. doi: 10.1002/jez.b.22941. Epub 2020 Mar 21. J Exp Zool B Mol Dev Evol. 2021. PMID: 32198827 Review.
-
The evolution of self during the transition to multicellularity.Adv Exp Med Biol. 2012;738:14-30. doi: 10.1007/978-1-4614-1680-7_2. Adv Exp Med Biol. 2012. PMID: 22399371 Review.
Cited by
-
The Saint-Leonard Urban Glaciotectonic Cave Harbors Rich and Diverse Planktonic and Sedimentary Microbial Communities.Microorganisms. 2024 Aug 29;12(9):1791. doi: 10.3390/microorganisms12091791. Microorganisms. 2024. PMID: 39338466 Free PMC article.
-
Cellular arrangement impacts metabolic activity and antibiotic tolerance in Pseudomonas aeruginosa biofilms.PLoS Biol. 2024 Feb 1;22(2):e3002205. doi: 10.1371/journal.pbio.3002205. eCollection 2024 Feb. PLoS Biol. 2024. PMID: 38300958 Free PMC article.
-
Cell arrangement impacts metabolic activity and antibiotic tolerance in Pseudomonas aeruginosa biofilms.bioRxiv [Preprint]. 2023 Aug 28:2023.06.20.545666. doi: 10.1101/2023.06.20.545666. bioRxiv. 2023. Update in: PLoS Biol. 2024 Feb 1;22(2):e3002205. doi: 10.1371/journal.pbio.3002205. PMID: 37645902 Free PMC article. Updated. Preprint.
-
A qualitative assessment of limits of active flight in low density atmospheres.Sci Rep. 2024 Jun 15;14(1):13823. doi: 10.1038/s41598-024-64114-4. Sci Rep. 2024. PMID: 38879676 Free PMC article.
-
Illuminating a new path to multicellularity.Elife. 2022 Oct 11;11:e83296. doi: 10.7554/eLife.83296. Elife. 2022. PMID: 36217823 Free PMC article.
References
-
- Amard B, Bertrand-Sarfati J. Microfossils in 2000 ma old cherty stromatolites of the franceville group, gabon. Precambrian Research. 1997;81:197–221. doi: 10.1016/S0301-9268(96)00035-6. - DOI
-
- Barton HA, Northup DE. Geomicrobiology in cave environments: past, current and future perspectives. Journal of Cave and Karst Studies. 2007;69:163–178.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

