Cannabinoid signaling modulation through JZL184 restores key phenotypes of a mouse model for Williams-Beuren syndrome
- PMID: 36217821
- PMCID: PMC9553213
- DOI: 10.7554/eLife.72560
Cannabinoid signaling modulation through JZL184 restores key phenotypes of a mouse model for Williams-Beuren syndrome
Abstract
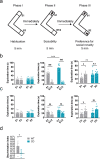
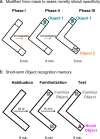
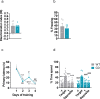
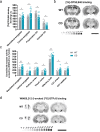
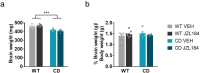
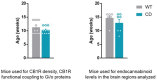
Williams-Beuren syndrome (WBS) is a rare genetic multisystemic disorder characterized by mild-to-moderate intellectual disability and hypersocial phenotype, while the most life-threatening features are cardiovascular abnormalities. Nowadays, there are no pharmacological treatments to directly ameliorate the main traits of WBS. The endocannabinoid system (ECS), given its relevance for both cognitive and cardiovascular function, could be a potential druggable target in this syndrome. We analyzed the components of the ECS in the complete deletion (CD) mouse model of WBS and assessed the impact of its pharmacological modulation in key phenotypes relevant for WBS. CD mice showed the characteristic hypersociable phenotype with no preference for social novelty and poor short-term object-recognition performance. Brain cannabinoid type-1 receptor (CB1R) in CD male mice showed alterations in density and coupling with no detectable change in main endocannabinoids. Endocannabinoid signaling modulation with subchronic (10 days) JZL184, a selective inhibitor of monoacylglycerol lipase, specifically normalized the social and cognitive phenotype of CD mice. Notably, JZL184 treatment improved cardiovascular function and restored gene expression patterns in cardiac tissue. These results reveal the modulation of the ECS as a promising novel therapeutic approach to improve key phenotypic alterations in WBS.
Keywords: Williams–Beuren syndrome; cannabinoid type-1 receptor; endocannabinoid system; intellectual disability; medicine; mouse.
Plain language summary
Williams-Beuren syndrome (WBS) is a rare disorder that causes hyper-social behavior, intellectual disability, memory problems, and life-threatening overgrowth of the heart. Behavioral therapies can help improve the cognitive and social aspects of the syndrome and surgery is sometimes used to treat the effects on the heart, although often with limited success. However, there are currently no medications available to treat WBS. The endocannabinoid system – which consists of cannabis-like chemical messengers that bind to specific cannabinoid receptor proteins – has been shown to influence cognitive and social behaviors, as well as certain functions of the heart. This has led scientists to suspect that the endocannabinoid system may play a role in WBS, and drugs modifying this network of chemical messengers could help treat the rare condition. To investigate, Navarro-Romero, Galera-López et al. studied mice which had the same genetic deletion found in patients with WBS. Similar to humans, the male mice displayed hyper-social behaviors, had memory deficits and enlarged hearts. Navarro-Romero, Galera-López et al. found that these mutant mice also had differences in the function of the receptor protein cannabinoid type-1 (CB1). The genetically modified mice were then treated with an experimental drug called JZL184 that blocks the breakdown of endocannabinoids which bind to the CB1 receptor. This normalized the number and function of receptors in the brains of the WBS mice, and reduced their social and memory symptoms. The treatment also restored the animals’ heart cells to a more normal size, improved the function of their heart tissue, and led to lower blood pressure. Further experiments revealed that the drug caused the mutant mice to activate many genes in their heart muscle cells to the same level as normal, healthy mice. These findings suggest that JZL184 or other drugs targeting the endocannabinoid system may help ease the symptoms associated with WBS. More studies are needed to test the drug’s effectiveness in humans with this syndrome. Furthermore, the dramatic effect JZL184 has on the heart suggests that it might also help treat high blood pressure or conditions that cause the overgrowth of heart cells.
© 2022, Navarro-Romero, Galera-López et al.
Conflict of interest statement
AN, LG, PO, AL, Ld, IB, AG, AM, MR, AP, Rd, RM, BB, RR, VC, AO No competing interests declared, EE Reviewing editor, eLife
Figures
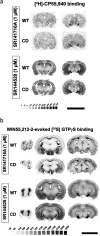
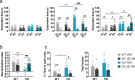


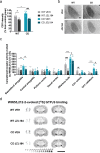

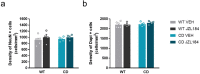


References
-
- Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, Offertáler L, Mackie K, Rudd MA, Bukoski RD, Kunos G. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. - DOI - PMC - PubMed
-
- Busquets-Garcia A, Gomis-González M, Guegan T, Agustín-Pavón C, Pastor A, Mato S, Pérez-Samartín A, Matute C, de la Torre R, Dierssen M, Maldonado R, Ozaita A. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nature Medicine. 2013;19:603–607. doi: 10.1038/nm.3127. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions

