Methods for drug safety signal detection using routinely collected observational electronic health care data: A systematic review
- PMID: 36218170
- PMCID: PMC10092128
- DOI: 10.1002/pds.5548
Methods for drug safety signal detection using routinely collected observational electronic health care data: A systematic review
Abstract
Purpose: Signal detection is a crucial step in the discovery of post-marketing adverse drug reactions. There is a growing interest in using routinely collected data to complement established spontaneous report analyses. This work aims to systematically review the methods for drug safety signal detection using routinely collected healthcare data and their performance, both in general and for specific types of drugs and outcomes.
Methods: We conducted a systematic review following the PRISMA guidelines, and registered a protocol in PROSPERO. MEDLINE, EMBASE, PubMed, Web of Science, Scopus, and the Cochrane Library were searched until July 13, 2021.
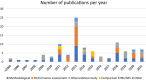
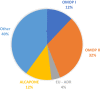
Results: The review included 101 articles, among which there were 39 methodological works, 25 performance assessment papers, and 24 observational studies. Methods included adaptations from those used with spontaneous reports, traditional epidemiological designs, methods specific to signal detection with real-world data. More recently, implementations of machine learning have been studied in the literature. Twenty-five studies evaluated method performances, 16 of them using the area under the curve (AUC) for a range of positive and negative controls as their main measure. Despite the likelihood that performance measurement could vary by drug-event pair, only 10 studies reported performance stratified by drugs and outcomes, in a heterogeneous manner. The replicability of the performance assessment results was limited due to lack of transparency in reporting and the lack of a gold standard reference set.
Conclusions: A variety of methods have been described in the literature for signal detection with routinely collected data. No method showed superior performance in all papers and across all drugs and outcomes, performance assessment and reporting were heterogeneous. However, there is limited evidence that self-controlled designs, high dimensional propensity scores, and machine learning can achieve higher performances than other methods.
Keywords: drug safety surveillance; pharmacoepidemiology; pharmacovigilance; real world data; signal detection; systematic review.
© 2022 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
Astrid Coste is funded by a GSK PhD studentship to undertake this review. Andrew Bate is an employee of GSK and holds stocks and stock options. Ian Douglas holds grants and shares from GSK.
Figures

References
-
- CIOMS . Working Group VIII. Practical Aspects of Signal Detection in Pharmacovigilance. Council for International Organizations of Medical Sciences (CIOMS); 2010.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

