Myosin 5b is required for proper localization of the intermicrovillar adhesion complex in the intestinal brush border
- PMID: 36218265
- PMCID: PMC9639760
- DOI: 10.1152/ajpgi.00212.2022
Myosin 5b is required for proper localization of the intermicrovillar adhesion complex in the intestinal brush border
Abstract
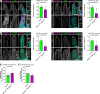
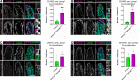
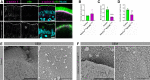
Intestinal enterocytes have an elaborate apical membrane of actin-rich protrusions known as microvilli. The organization of microvilli is orchestrated by the intermicrovillar adhesion complex (IMAC), which connects the distal tips of adjacent microvilli. The IMAC is composed of CDHR2 and CDHR5 as well as the scaffolding proteins USH1C, ANKS4B, and Myosin 7b (MYO7B). To create an IMAC, cells must transport the proteins to the apical membrane. Myosin 5b (MYO5B) is a molecular motor that traffics ion transporters to the apical membrane of enterocytes, and we hypothesized that MYO5B may also be responsible for the localization of IMAC proteins. To address this question, we used two different mouse models: 1) neonatal germline MYO5B knockout (MYO5B KO) mice and 2) adult intestinal-specific tamoxifen-inducible VillinCreERT2;MYO5Bflox/flox mice. In control mice, immunostaining revealed that CDHR2, CDHR5, USH1C, and MYO7B were highly enriched at the tips of the microvilli. In contrast, neonatal germline and adult MYO5B-deficient mice showed loss of apical CDHR2, CDHR5, and MYO7B in the brush border and accumulation in a subapical compartment. Colocalization analysis revealed decreased Mander's coefficients in adult inducible MYO5B-deficient mice compared with control mice for CDHR2, CDHR5, USH1C, and MYO7B. Scanning electron microscopy images further demonstrated aberrant microvilli packing in adult inducible MYO5B-deficient mouse small intestine. These data indicate that MYO5B is responsible for the delivery of IMAC components to the apical membrane.NEW & NOTEWORTHY The intestinal epithelium absorbs nutrients and water through an elaborate apical membrane of highly organized microvilli. Microvilli organization is regulated by the intermicrovillar adhesion complexes, which create links between neighboring microvilli and control microvilli packing and density. In this study, we report a new trafficking partner of the IMAC, Myosin 5b. Loss of Myosin 5b results in a disorganized brush border and failure of IMAC proteins to reach the distal tips of microvilli.
Keywords: enterocyte; intermicrovillar adhesion complex (IMAC); intestine; microvilli; myosin.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Similar articles
-
A heterologous in-cell assay for investigating intermicrovillar adhesion complex interactions reveals a novel protrusion length-matching mechanism.J Biol Chem. 2020 Nov 27;295(48):16191-16206. doi: 10.1074/jbc.RA120.015929. Epub 2020 Oct 13. J Biol Chem. 2020. PMID: 33051206 Free PMC article.
-
Myosin-7b Promotes Distal Tip Localization of the Intermicrovillar Adhesion Complex.Curr Biol. 2016 Oct 24;26(20):2717-2728. doi: 10.1016/j.cub.2016.08.014. Epub 2016 Sep 22. Curr Biol. 2016. PMID: 27666969 Free PMC article.
-
Loss of MYO5B Leads to Reductions in Na+ Absorption With Maintenance of CFTR-Dependent Cl- Secretion in Enterocytes.Gastroenterology. 2018 Dec;155(6):1883-1897.e10. doi: 10.1053/j.gastro.2018.08.025. Epub 2018 Aug 23. Gastroenterology. 2018. PMID: 30144427 Free PMC article.
-
Trafficking Ion Transporters to the Apical Membrane of Polarized Intestinal Enterocytes.Cold Spring Harb Perspect Biol. 2018 Jan 2;10(1):a027979. doi: 10.1101/cshperspect.a027979. Cold Spring Harb Perspect Biol. 2018. PMID: 28264818 Free PMC article. Review.
-
Altered MYO5B Function Underlies Microvillus Inclusion Disease: Opportunities for Intervention at a Cellular Level.Cell Mol Gastroenterol Hepatol. 2022;14(3):553-565. doi: 10.1016/j.jcmgh.2022.04.015. Epub 2022 Jun 1. Cell Mol Gastroenterol Hepatol. 2022. PMID: 35660026 Free PMC article. Review.
Cited by
-
Injured Proximal Tubular Epithelial Cells Lose Hepatocyte Nuclear Factor 4α Expression Crucial for Brush Border Formation and Transport.Am J Pathol. 2025 May;195(5):845-860. doi: 10.1016/j.ajpath.2025.01.011. Epub 2025 Feb 13. Am J Pathol. 2025. PMID: 39954965 Free PMC article.
-
Paraffin Embedding and Histological Staining of Intestinal Organoids.Methods Mol Biol. 2025;2951:35-45. doi: 10.1007/7651_2025_633. Methods Mol Biol. 2025. PMID: 40459836
-
Optimized Protocol for Intestinal Swiss Rolls and Immunofluorescent Staining of Paraffin Embedded Tissue.J Vis Exp. 2024 Jul 19;(209):10.3791/66977. doi: 10.3791/66977. J Vis Exp. 2024. PMID: 39141543 Free PMC article.
-
E-Cadherin Is a Structuring Component of Invadopodia in Pancreatic Cancer.J Cell Mol Med. 2025 May;29(9):e70608. doi: 10.1111/jcmm.70608. J Cell Mol Med. 2025. PMID: 40366255 Free PMC article.
-
The Molecular Motor Myosin 5B and Its Folding Chaperone UNC45A Are Decreased in Colorectal Cancer.Cell Mol Gastroenterol Hepatol. 2025;19(9):101537. doi: 10.1016/j.jcmgh.2025.101537. Epub 2025 May 21. Cell Mol Gastroenterol Hepatol. 2025. PMID: 40409685 Free PMC article.
References
-
- Crawley SW, Shifrin DA Jr, Grega-Larson NE, McConnell RE, Benesh AE, Mao S, Zheng Y, Zheng QY, Nam KT, Millis BA, Kachar B, Tyska MJ. Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell 157: 433–446, 2014. doi:10.1016/j.cell.2014.01.067. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

