E-cigarette vapor exposure in utero causes long-term pulmonary effects in offspring
- PMID: 36218276
- PMCID: PMC9722245
- DOI: 10.1152/ajplung.00233.2022
E-cigarette vapor exposure in utero causes long-term pulmonary effects in offspring
Abstract
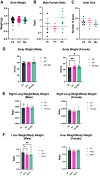
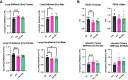
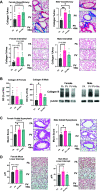
The in utero environment is sensitive to toxicant exposure, altering the health and growth of the fetus, and thus sensitive to contaminant exposure. Though recent clinical data suggest that e-cigarette use does no further harm to birth outcomes than a nicotine patch, this does not account for the effects of vaping during pregnancy on the long-term health of offspring. Pregnant mice were exposed to: 1) e-cigarette vapor with nicotine (PV + Nic; 2% Nic in 50:50 propylene glycol: vegetable glycerin), 2) e-cigarette vapor without nicotine [PV; (50:50 propylene glycol:vegetable glycerin)], or 3) HEPA filtered air (FA). Dams were removed from exposure upon giving birth. At 5 mo of age, pulmonary function tests on the offspring revealed female and male mice from the PV group had greater lung stiffness (Ers) and alveolar stiffness (H) compared with the FA group. Furthermore, baseline compliance (Crs) was reduced in female mice from the PV group and in male mice from the PV and PV + Nic groups. Lastly, female mice had decreased forced expiratory volume (FEV0.1) in the PV group, but not in the male groups, compared with the FA group. Lung histology revealed increased collagen deposition around the vessels/airways and in alveolar tissue in PV and PV + Nic groups. Furthermore, goblet hyperplasia was observed in PV male and PV/PV + Nic female mice. Our work shows that in utero exposure to e-cigarette vapor, regardless of nicotine presence, causes lung dysfunction and structural impairments that persist in the offspring to adulthood.
Keywords: e-cigarette; fibrosis; in utero; pulmonary; vaping.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Canistro D, Vivarelli F, Cirillo S, Babot Marquillas C, Buschini A, Lazzaretti M, Marchi L, Cardenia V, Rodriguez-Estrada MT, Lodovici M, Cipriani C, Lorenzini A, Croco E, Marchionni S, Franchi P, Lucarini M, Longo V, della Croce CM, Vornoli A, Colacci A, Vaccari M, Sapone A, Paolini M. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci Rep 7: 2028, 2017. doi:10.1038/s41598-017-02317-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

