Structure-Guided Discovery of Potent Antifungals that Prevent Ras Signaling by Inhibiting Protein Farnesyltransferase
- PMID: 36218371
- PMCID: PMC10755971
- DOI: 10.1021/acs.jmedchem.2c00902
Structure-Guided Discovery of Potent Antifungals that Prevent Ras Signaling by Inhibiting Protein Farnesyltransferase
Abstract
Infections by fungal pathogens are difficult to treat due to a paucity of antifungals and emerging resistances. Next-generation antifungals therefore are needed urgently. We have developed compounds that prevent farnesylation of Cryptoccoccus neoformans Ras protein by inhibiting protein farnesyltransferase with 3-4 nanomolar affinities. Farnesylation directs Ras to the cell membrane and is required for infectivity of this lethal pathogenic fungus. Our high-affinity compounds inhibit fungal growth with 3-6 micromolar minimum inhibitory concentrations (MICs), 4- to 8-fold better than Fluconazole, an antifungal commonly used in the clinic. Compounds bound with distinct inhibition mechanisms at two alternative, partially overlapping binding sites, accessed via different inhibitor conformations. We showed that antifungal potency depends critically on the selected inhibition mechanism because this determines the efficacy of an inhibitor at low in vivo levels of enzyme and farnesyl substrate. We elucidated how chemical modifications of the antifungals encode desired inhibitor conformation and concomitant inhibitory mechanism.
Figures
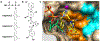







References
-
- Schmiedel Y; Zimmerli S Common invasive fungal diseases: an overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med. Wkly 2016, 146, w14281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

