SARS-CoV-2-Neutralizing Humoral IgA Response Occurs Earlier but Is Modest and Diminishes Faster than IgG Response
- PMID: 36219096
- PMCID: PMC9769934
- DOI: 10.1128/spectrum.02716-22
SARS-CoV-2-Neutralizing Humoral IgA Response Occurs Earlier but Is Modest and Diminishes Faster than IgG Response
Abstract
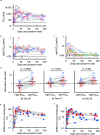
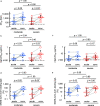
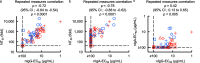
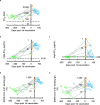
Secretory immunoglobulin A (IgA) plays a crucial role in mucosal immunity for preventing the invasion of exogenous antigens; however, little is understood about the neutralizing activity of serum IgA. Here, to examine the role of IgA antibodies against COVID-19 illnesses, we determined the neutralizing activity of serum/plasma IgG and IgA purified from previously SARS-CoV-2-infected and COVID-19 mRNA vaccine-receiving individuals. We found that serum/plasma IgA possesses substantial but rather modest neutralizing activity against SARS-CoV-2 compared to IgG with no significant correlation with the disease severity. Neutralizing IgA and IgG antibodies achieved the greatest activity at approximately 25 and 35 days after symptom onset, respectively. However, neutralizing IgA activity quickly diminished to below the detection limit approximately 70 days after onset, while substantial IgG activity was observed until 200 days after onset. The total neutralizing activity in sera/plasmas of those with COVID-19 largely correlated with those in purified IgG and purified IgA and levels of anti-SARS-CoV-2-S1-binding IgG and anti-SARS-CoV-2-S1-binding IgA. In individuals who were previously infected with SARS-CoV-2 but had no detectable neutralizing IgA activity, a single dose of BNT162b2 or mRNA-1273 elicited potent serum/plasma-neutralizing IgA activity, but the second dose did not further strengthen the neutralization antibody response. The present data show that the systemic immune stimulation with natural infection and COVID-19 mRNA-vaccines elicits both SARS-CoV-2-specific neutralizing IgG and IgA responses in serum, but the IgA response is modest and diminishes faster than the IgG response. IMPORTANCE Secretory dimeric immunoglobulin A (IgA) plays an important role in preventing the invasion of foreign objects by its neutralizing activity on mucosal surfaces, while monomeric serum IgA is thought to relate to the phagocytic immune system activation. Here, we report that individuals with the novel coronavirus disease (COVID-19) developed both systemic neutralizing IgG (nIgG) and IgA (nIgA) active against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although the nIgA response was quick and reached the highest activity earlier than the nIgG response, nIgA activity was modest and diminished faster than nIgG activity. In individuals who recovered from COVID-19 but had no detectable nIgA activity, a single dose of COVID-19 mRNA vaccine elicited potent nIgA activity, but the second dose did not further strengthen the antibody response. Our study provides novel insights into the role and the kinetics of serum nIgA against the pathogen in both naturally infected and COVID-19 mRNA vaccine-receiving COVID-19-convalescent individuals.
Keywords: COVID-19; COVID-19 mRNA vaccine; SARS-CoV-2; anti-SARS-CoV-2 immunoglobulin; humoral immunity; immunoglobulin A; immunoglobulin G; neutralizing antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Update of
-
SARS-CoV-2-neutralizing humoral IgA response occurs earlier but modest and diminishes faster compared to IgG response.bioRxiv [Preprint]. 2022 Jun 9:2022.06.09.495422. doi: 10.1101/2022.06.09.495422. bioRxiv. 2022. Update in: Microbiol Spectr. 2022 Dec 21;10(6):e0271622. doi: 10.1128/spectrum.02716-22. PMID: 35702154 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

