Cholesterol Stiffening of Lipid Membranes
- PMID: 36219221
- PMCID: PMC9552730
- DOI: 10.1007/s00232-022-00263-9
Cholesterol Stiffening of Lipid Membranes
Abstract
Biomembrane order, dynamics, and other essential physicochemical parameters are controlled by cholesterol, a major component of mammalian cell membranes. Although cholesterol is well known to exhibit a condensing effect on fluid lipid membranes, the extent of stiffening that occurs with different degrees of lipid acyl chain unsaturation remains an enigma. In this review, we show that cholesterol locally increases the bending rigidity of both unsaturated and saturated lipid membranes, suggesting there may be a length-scale dependence of the bending modulus. We review our published data that address the origin of the mechanical effects of cholesterol on unsaturated and polyunsaturated lipid membranes and their role in biomembrane functions. Through a combination of solid-state deuterium NMR spectroscopy and neutron spin-echo spectroscopy, we show that changes in molecular packing cause the universal effects of cholesterol on the membrane bending rigidity. Our findings have broad implications for the role of cholesterol in lipid-protein interactions as well as raft-like mixtures, drug delivery applications, and the effects of antimicrobial peptides on lipid membranes.
Keywords: Area per lipid; Cholesterol; Membrane elasticity; Neutron spin-echo; Rafts; Solid-state NMR spectroscopy.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
The authors declare that they have no conflicts of interest.
Figures

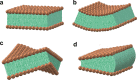
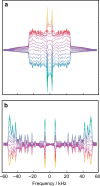
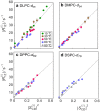
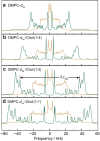


References
-
- Arfken G. Mathematical methods for physicsts. New York: Academic Press; 1970.
-
- Arriaga LR, López-Montero I, Monroy F, Orts-Gil G, Hellweg T. Stiffening effect of cholesterol on disordered lipid phases: a combined neutron spin echo + dynamic light scattering analysis of the bending elasticity of large unilamellar vesicles. Biophys J. 2009;96:3629–3637. doi: 10.1016/j.bpj.2009.01.045. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

