Chiral-at-Metal Silver-Mediated Base Pairs: Metal-Centred Chirality versus DNA Helical Chirality
- PMID: 36219466
- PMCID: PMC10098492
- DOI: 10.1002/chem.202202630
Chiral-at-Metal Silver-Mediated Base Pairs: Metal-Centred Chirality versus DNA Helical Chirality
Abstract
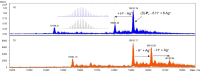
When covalently incorporating ligands capable of forming chiral metal complexes into a DNA oligonucleotide duplex, an enantiospecific formation of metal-mediated base pairs is possible. We have been investigating the chirality of the silver-mediated base pair P-AgI -P (P, 1H-imidazo[4,5-f][1,10]phenanthroline) depending on the number of consecutive P : P pairs within a series of duplexes. Towards this end, both enantiomers of the nucleoside analogue 3-(1H-imidazo[4,5-f][1,10]phenanthrolin-1-yl)propane-1,2-diol comprising an acyclic backbone were introduced into DNA duplexes, resulting in diastereomeric metal-mediated base pairs. The same chiral-at-metal complex is formed inside the duplex for up to five neighbouring P-AgI -P pairs, irrespective of whether (S)-P or (R)-P is used. With six silver-mediated base pairs, the chirality of the metal complex is inverted for (S)-P but not for (R)-P. This indicates an intricate balance of what determines the configuration of the metal complex, the intrinsically preferred metal-centred chirality or the DNA helical chirality.
Keywords: Chirality; DNA; metal-mediated base pair; phenanthroline; silver.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

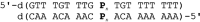


Similar articles
-
Contiguous Silver(I)-Mediated Base Pairs of Imidazophenanthroline and Canonical Nucleobases in DNA Duplexes: Formation of Classical Duplexes versus Homodimer Formation.Bioconjug Chem. 2024 Jan 17;35(1):99-106. doi: 10.1021/acs.bioconjchem.3c00494. Epub 2023 Dec 29. Bioconjug Chem. 2024. PMID: 38157473
-
A stable zinc(II)-mediated base pair in a parallel-stranded DNA duplex.J Inorg Biochem. 2018 Sep;186:301-306. doi: 10.1016/j.jinorgbio.2018.07.002. Epub 2018 Jul 5. J Inorg Biochem. 2018. PMID: 30005358
-
Sequence-Dependent Duplex Stabilization upon Formation of a Metal-Mediated Base Pair.Chemistry. 2016 Jan 4;22(1):295-301. doi: 10.1002/chem.201503405. Epub 2015 Nov 20. Chemistry. 2016. PMID: 26584591
-
Binding of metal ions by pyrimidine base pairs in DNA duplexes.Chem Soc Rev. 2011 Dec;40(12):5855-66. doi: 10.1039/c1cs15149e. Epub 2011 Aug 8. Chem Soc Rev. 2011. PMID: 21826352 Review.
-
Alternative DNA base pairing through metal coordination.Met Ions Life Sci. 2012;10:269-94. doi: 10.1007/978-94-007-2172-2_10. Met Ions Life Sci. 2012. PMID: 22210343 Review.
Cited by
-
6-Pyrazolylpurine and its deaza derivatives as nucleobases for silver(I)-mediated base pairing with pyrimidines.J Biol Inorg Chem. 2023 Dec;28(8):791-803. doi: 10.1007/s00775-023-02022-0. Epub 2023 Nov 20. J Biol Inorg Chem. 2023. PMID: 37982840 Free PMC article.
References
-
- None
-
- Naskar S., Guha R., Müller J., Angew. Chem. Int. Ed. 2020, 59, 1397–1406; - PubMed
- Angew. Chem. 2020, 132, 1413–1423;
-
- Takezawa Y., Müller J., Shionoya M., Chem. Lett. 2017, 46, 622–633;
-
- Tanaka Y., Kondo J., Sychrovský V., Šebera J., Dairaku T., Saneyoshi H., Urata H., Torigoe H., Ono A., Chem. Commun. 2015, 51, 17343–17360; - PubMed
-
- Clever G. H., Kaul C., Carell T., Angew. Chem. Int. Ed. 2007, 46, 6226–6236; - PubMed
- Angew. Chem. 2007, 119, 6340–6350.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

