Construction of an rAAV Producer Cell Line through Synthetic Biology
- PMID: 36219557
- PMCID: PMC9595119
- DOI: 10.1021/acssynbio.2c00207
Construction of an rAAV Producer Cell Line through Synthetic Biology
Abstract
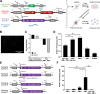
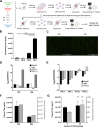
Recombinant adeno-associated viruses (rAAV) are important gene delivery vehicles for gene therapy applications. Their production relies on plasmid transfection or virus infection of producer cells, which pose a challenge in process scale-up. Here, we describe a template for a transfection-free, helper virus-free rAAV producer cell line using a synthetic biology approach. Three modules were integrated into HEK293 cells including an rAAV genome and multiple inducible promoters controlling the expression of AAV Rep, Cap, and helper coding sequences. The synthetic cell line generated infectious rAAV vectors upon induction. Independent control over replication and packaging activities allowed for manipulation of the fraction of capsid particles containing viral genomes, affirming the feasibility of tuning gene expression profiles in a synthetic cell line for enhancing the quality of the viral vector produced. The synthetic biology approach for rAAV production presented in this study can be exploited for scalable biomanufacturing.
Keywords: HEK293; adeno-associated virus; biomanufacturing; synthetic biology.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Recombinant adeno-associated virus type 2 replication and packaging is entirely supported by a herpes simplex virus type 1 amplicon expressing Rep and Cap.J Virol. 1997 Nov;71(11):8780-9. doi: 10.1128/JVI.71.11.8780-8789.1997. J Virol. 1997. PMID: 9343238 Free PMC article.
-
A synthetic platform for developing recombinant adeno-associated virus type 8 producer cell lines.Biotechnol Prog. 2025 May-Jun;41(3):e70009. doi: 10.1002/btpr.70009. Epub 2025 Feb 19. Biotechnol Prog. 2025. PMID: 39968660 Free PMC article.
-
Comparative transcriptomic and proteomic kinetic analysis of adeno-associated virus production systems.Appl Microbiol Biotechnol. 2024 Jun 19;108(1):385. doi: 10.1007/s00253-024-13203-5. Appl Microbiol Biotechnol. 2024. PMID: 38896252 Free PMC article.
-
Production of recombinant adeno-associated virus vectors.Hum Gene Ther. 2005 May;16(5):551-7. doi: 10.1089/hum.2005.16.551. Hum Gene Ther. 2005. PMID: 15916480 Review.
-
Recombinant adeno-associated virus as delivery vector for gene therapy--a review.Stem Cells Dev. 2004 Feb;13(1):133-45. doi: 10.1089/154732804773099335. Stem Cells Dev. 2004. PMID: 15068701 Review.
Cited by
-
Unlocking DOE potential by selecting the most appropriate design for rAAV optimization.Mol Ther Methods Clin Dev. 2024 Aug 26;32(4):101329. doi: 10.1016/j.omtm.2024.101329. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39296857 Free PMC article.
-
AAV production in stable packaging cells requires expression of adenovirus 22/33K protein to allow episomal amplification of integrated rep/cap genes.Sci Rep. 2023 Dec 7;13(1):21670. doi: 10.1038/s41598-023-48901-z. Sci Rep. 2023. PMID: 38066084 Free PMC article.
-
High-purity AAV vector production utilizing recombination-dependent minicircle formation and genetic coupling.EMBO Mol Med. 2025 Jun;17(6):1475-1494. doi: 10.1038/s44321-025-00248-w. Epub 2025 May 16. EMBO Mol Med. 2025. PMID: 40379974 Free PMC article.
-
Synthetic Cell Lines for Inducible Packaging of Influenza A Virus.ACS Synth Biol. 2024 Feb 16;13(2):546-557. doi: 10.1021/acssynbio.3c00526. Epub 2024 Jan 23. ACS Synth Biol. 2024. PMID: 38259154 Free PMC article.
-
Engineering an Autonucleolytic Mammalian Suspension Host Cell Line to Reduce DNA Impurity Levels in Serum-Free Lentiviral Process Streams.ACS Synth Biol. 2024 Feb 16;13(2):466-473. doi: 10.1021/acssynbio.3c00682. Epub 2024 Jan 24. ACS Synth Biol. 2024. PMID: 38266181 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

