Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study
- PMID: 36219809
- PMCID: PMC9902012
- DOI: 10.1200/JCO.21.02508
Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study
Abstract
Purpose: Pembrolizumab and pembrolizumab-chemotherapy demonstrated efficacy in recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048. Post hoc analysis of long-term efficacy and progression-free survival on next-line therapy (PFS2) is presented.
Methods: Patients were randomly assigned (1:1:1) to pembrolizumab, pembrolizumab-chemotherapy, or cetuximab-chemotherapy. Efficacy was evaluated in programmed death ligand 1 (PD-L1) combined positive score (CPS) ≥ 20, CPS ≥ 1, and total populations, with no multiplicity or alpha adjustment.
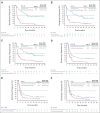
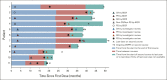
Results: The median study follow-up was 45.0 months (interquartile range, 41.0-49.2; n = 882). At data cutoff (February 18, 2020), overall survival improved with pembrolizumab in the PD-L1 CPS ≥ 20 (hazard ratio [HR], 0.61; 95% CI, 0.46 to 0.81) and CPS ≥ 1 populations (HR, 0.74; 95% CI, 0.61 to 0.89) and was noninferior in the total population (HR, 0.81; 95% CI, 0.68 to 0.97). Overall survival improved with pembrolizumab-chemotherapy in the PD-L1 CPS ≥ 20 (HR, 0.62; 95% CI, 0.46 to 0.84), CPS ≥ 1 (HR, 0.64; 95% CI, 0.53 to 0.78), and total (HR, 0.71; 95% CI, 0.59 to 0.85) populations. The objective response rate on second-course pembrolizumab was 27.3% (3 of 11). PFS2 improved with pembrolizumab in the PD-L1 CPS ≥ 20 (HR, 0.64; 95% CI, 0.48 to 0.84) and CPS ≥ 1 (HR, 0.79; 95% CI, 0.66 to 0.95) populations and with pembrolizumab-chemotherapy in the PD-L1 CPS ≥ 20 (HR, 0.64; 95% CI, 0.48 to 0.86), CPS ≥ 1 (HR, 0.66; 95% CI, 0.55 to 0.81), and total (HR, 0.73; 95% CI, 0.61 to 0.88) populations. PFS2 was similar after pembrolizumab and longer after pembrolizumab-chemotherapy on next-line taxanes and shorter after pembrolizumab and similar after pembrolizumab-chemotherapy on next-line nontaxanes.
Conclusion: With a 4-year follow-up, first-line pembrolizumab and pembrolizumab-chemotherapy continued to demonstrate survival benefit versus cetuximab-chemotherapy in recurrent/metastatic head and neck squamous cell carcinoma. Patients responded well to subsequent treatment after pembrolizumab-based therapy.
Trial registration: ClinicalTrials.gov NCT02358031.
Conflict of interest statement
This author is an Associate Editor for
No other potential conflicts of interest were reported.
Figures




Comment in
-
Long-term efficacy of immune checkpoint inhibitors with or without chemotherapy in recurrent or metastatic squamous cell carcinoma of the head and neck: a commentary on the 4-year follow-up of the KEYNOTE-048 trial.Transl Cancer Res. 2023 May 31;12(5):1363-1367. doi: 10.21037/tcr-23-48. Epub 2023 Apr 14. Transl Cancer Res. 2023. PMID: 37304541 Free PMC article. No abstract available.
References
-
- Chow LQM: Head and neck cancer. N Engl J Med 382:60-72, 2020 - PubMed
-
- Burtness B, Harrington KJ, Greil R, et al. : Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394:1915-1928, 2019 - PubMed
-
- Cohen EEW, Soulieres D, Le Tourneau C, et al. : Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 393:156-167, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

