RUNX1-deficient human megakaryocytes demonstrate thrombopoietic and platelet half-life and functional defects
- PMID: 36219879
- PMCID: PMC9936297
- DOI: 10.1182/blood.2022017561
RUNX1-deficient human megakaryocytes demonstrate thrombopoietic and platelet half-life and functional defects
Abstract
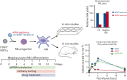
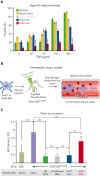
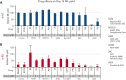
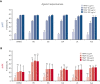
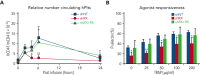
Heterozygous defects in runt-related transcription factor 1 (RUNX1) are causative of a familial platelet disorder with associated myeloid malignancy (FPDMM). Because RUNX1-deficient animal models do not mimic bleeding disorder or leukemic risk associated with FPDMM, development of a proper model system is critical to understanding the underlying mechanisms of the observed phenotype and to identifying therapeutic interventions. We previously reported an in vitro megakaryopoiesis system comprising human CD34+ hematopoietic stem and progenitor cells that recapitulated the FPDMM quantitative megakaryocyte defect through a decrease in RUNX1 expression via a lentiviral short hairpin RNA strategy. We now show that shRX-megakaryocytes have a marked reduction in agonist responsiveness. We then infused shRX-megakaryocytes into immunocompromised NOD scid gamma (NSG) mice and demonstrated that these megakaryocytes released fewer platelets than megakaryocytes transfected with a nontargeting shRNA, and these platelets had a diminished half-life. The platelets were also poorly responsive to agonists, unable to correct thrombus formation in NSG mice homozygous for a R1326H mutation in von Willebrand Factor (VWFR1326H), which switches the species-binding specificity of the VWF from mouse to human glycoprotein Ibα. A small-molecule inhibitor RepSox, which blocks the transforming growth factor β1 (TGFβ1) pathway and rescued defective megakaryopoiesis in vitro, corrected the thrombopoietic defect, defects in thrombus formation and platelet half-life, and agonist response in NSG/VWFR1326H mice. Thus, this model recapitulates the defects in FPDMM megakaryocytes and platelets, identifies previously unrecognized defects in thrombopoiesis and platelet half-life, and demonstrates for the first time, reversal of RUNX1 deficiency-induced hemostatic defects by a drug.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



References
-
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84(2):321–330. - PubMed
-
- Wang Q, Stacy T, Miller JD, et al. The CBFbeta subunit is essential for CBFalpha2 (AML1) function in vivo. Cell. 1996;87(4):697–708. - PubMed
-
- Kalev-Zylinska ML, Horsfield JA, Flores MV, et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129(8):2015–2030. - PubMed
-
- Ichikawa M, Asai T, Saito T, et al. AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nat Med. 2004;10(3):299–304. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

