Single-cell spatial transcriptomics reveals a dynamic control of metabolic zonation and liver regeneration by endothelial cell Wnt2 and Wnt9b
- PMID: 36220068
- PMCID: PMC9588996
- DOI: 10.1016/j.xcrm.2022.100754
Single-cell spatial transcriptomics reveals a dynamic control of metabolic zonation and liver regeneration by endothelial cell Wnt2 and Wnt9b
Abstract
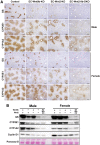
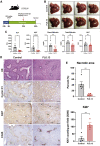
The conclusive identity of Wnts regulating liver zonation (LZ) and regeneration (LR) remains unclear despite an undisputed role of β-catenin. Using single-cell analysis, we identified a conserved Wnt2 and Wnt9b expression in endothelial cells (ECs) in zone 3. EC-elimination of Wnt2 and Wnt9b led to both loss of β-catenin targets in zone 3, and re-appearance of zone 1 genes in zone 3, unraveling dynamicity in the LZ process. Impaired LR observed in the knockouts phenocopied models of defective hepatic Wnt signaling. Administration of a tetravalent antibody to activate Wnt signaling rescued LZ and LR in the knockouts and induced zone 3 gene expression and LR in controls. Administration of the agonist also promoted LR in acetaminophen overdose acute liver failure (ALF) fulfilling an unmet clinical need. Overall, we report an unequivocal role of EC-Wnt2 and Wnt9b in LZ and LR and show the role of Wnt activators as regenerative therapy for ALF.
Keywords: Wnt signaling; acetaminophen; endothelial cells; hepatocyte; hepatocyte proliferation; liver injury; liver regeneration; metabolic zonation; repair; single cell spatial transcriptomics.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.P.M. is a consultant for Surrozen. L.L.B., J.J.A., S.S.S., and S.A. are shareholders of AntlerA Therapeutics.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

