Time-restricted eating with or without low-carbohydrate diet reduces visceral fat and improves metabolic syndrome: A randomized trial
- PMID: 36220069
- PMCID: PMC9589024
- DOI: 10.1016/j.xcrm.2022.100777
Time-restricted eating with or without low-carbohydrate diet reduces visceral fat and improves metabolic syndrome: A randomized trial
Abstract
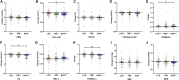
Overconsumption of carbohydrate-rich food combined with adverse eating patterns contributes to the increasing incidence of metabolic syndrome (MetS) in China. Therefore, we conducted a randomized trial to determine the effects of a low-carbohydrate diet (LCD), an 8-h time-restricted eating (TRE) schedule, and their combination on body weight and abdominal fat area (i.e., primary outcomes) and cardiometabolic outcomes in participants with MetS. Compared with baseline, all 3-month treatments significantly reduce body weight and subcutaneous fat area, but only TRE and combination treatment reduce visceral fat area (VFA), fasting blood glucose, uric acid (UA), and dyslipidemia. Furthermore, compared with changes of LCD, TRE and combination treatment further decrease body weight and VFA, while only combination treatment yields more benefits on glycemic control, UA, and dyslipidemia. In conclusion, without change of physical activity, an 8-h TRE with or without LCD can serve as an effective treatment for MetS (ClinicalTrials.gov: NCT04475822).
Keywords: abdominal obesity; low-carbohydrate diet; metabolic syndrome; time-restricted eating; visceral fat area.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Sperling L.S., Mechanick J.I., Neeland I.J., Herrick C.J., Després J.P., Ndumele C.E., Vijayaraghavan K., Handelsman Y., Puckrein G.A., Araneta M.R.G., et al. The CardioMetabolic health alliance: working toward a new care model for the metabolic syndrome. J. Am. Coll. Cardiol. 2015;66:1050–1067. - PubMed
-
- Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P.T., Loria C.M., Smith S.C., Jr., et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. - PubMed
-
- Wilson P.W.F., D'Agostino R.B., Parise H., Sullivan L., Meigs J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. - PubMed
-
- Zhao D., Liu J., Wang M., Zhang X., Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat. Rev. Cardiol. 2019;16:203–212. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

