Vaccine effectiveness of primary series and booster doses against covid-19 associated hospital admissions in the United States: living test negative design study
- PMID: 36220174
- PMCID: PMC9551237
- DOI: 10.1136/bmj-2022-072065
Vaccine effectiveness of primary series and booster doses against covid-19 associated hospital admissions in the United States: living test negative design study
Abstract
Objective: To compare the effectiveness of a primary covid-19 vaccine series plus booster doses with a primary series alone for the prevention of hospital admission with omicron related covid-19 in the United States.
Design: Multicenter observational case-control study with a test negative design.
Setting: Hospitals in 18 US states.
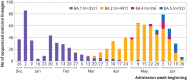
Participants: 4760 adults admitted to one of 21 hospitals with acute respiratory symptoms between 26 December 2021 and 30 June 2022, a period when the omicron variant was dominant. Participants included 2385 (50.1%) patients with laboratory confirmed covid-19 (cases) and 2375 (49.9%) patients who tested negative for SARS-CoV-2 (controls).
Main outcome measures: The main outcome was vaccine effectiveness against hospital admission with covid-19 for a primary series plus booster doses and a primary series alone by comparing the odds of being vaccinated with each of these regimens versus being unvaccinated among cases versus controls. Vaccine effectiveness analyses were stratified by immunosuppression status (immunocompetent, immunocompromised). The primary analysis evaluated all covid-19 vaccine types combined, and secondary analyses evaluated specific vaccine products.
Results: Overall, median age of participants was 64 years (interquartile range 52-75 years), 994 (20.8%) were immunocompromised, 85 (1.8%) were vaccinated with a primary series plus two boosters, 1367 (28.7%) with a primary series plus one booster, and 1875 (39.3%) with a primary series alone, and 1433 (30.1%) were unvaccinated. Among immunocompetent participants, vaccine effectiveness for prevention of hospital admission with omicron related covid-19 for a primary series plus two boosters was 63% (95% confidence interval 37% to 78%), a primary series plus one booster was 65% (58% to 71%), and for a primary series alone was 37% (25% to 47%) (P<0.001 for the pooled boosted regimens compared with a primary series alone). Vaccine effectiveness was higher for a boosted regimen than for a primary series alone for both mRNA vaccines (BNT162b2 (Pfizer-BioNTech): 73% (44% to 87%) for primary series plus two boosters, 64% (55% to 72%) for primary series plus one booster, and 36% (21% to 48%) for primary series alone (P<0.001); mRNA-1273 (Moderna): 68% (17% to 88%) for primary series plus two boosters, 65% (55% to 73%) for primary series plus one booster, and 41% (25% to 54%) for primary series alone (P=0.001)). Among immunocompromised patients, vaccine effectiveness for a primary series plus one booster was 69% (31% to 86%) and for a primary series alone was 49% (30% to 63%) (P=0.04).
Conclusion: During the first six months of 2022 in the US, booster doses of a covid-19 vaccine provided additional benefit beyond a primary vaccine series alone for preventing hospital admissions with omicron related covid-19.
Readers' note: This article is a living test negative design study that will be updated to reflect emerging evidence. Updates may occur for up to two years from the date of original publication.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: Funding for this work was provided to all participating sites by the US Centers for Disease Control and Prevention. SMB reports grants from the National Institutes of Health (NIH) and Department of Defense (DoD), participation as the Data Safety Monitoring Board (DSMB) chair for Hamilton Ventilators, and participation as a member of the DSMB for New York University covid-19 clinical trials. JDCasey reports funding from NIH and DoD. SYC reports consulting fees from La Jolla Pharmaceuticals, PureTech Health, and Kiniska Pharmaceuticals, payments or honorariums from La Jolla Pharmaceuticals, and participation on a DSMB for an investigator initiated study conducted at UCLA. JDChappell reports grants and other support from NIH. AD reports consulting fees from ALung technologies. MCE reports payments or honorariums from Abbott Laboratory for sponsored talks. DCF reports consulting fees from Cytovale and participation on a DSMB for Medpace. AEF reports grants from NIH. MG reports grants from CDC, CDC-Abt Associates, CDC-Westat, and Janssen, and a leadership role as co-chair of the Infectious Disease and Immunization Committee of the Texas Pediatric Society, Texas Chapter of American Academy of Pediatrics. KWG reports funding from NIH/National Heart, Lung, and Blood Institute (NHLBI) for the ACTIV-4HT NECTAR trial. AAG reports grants from NIH, DoD, AbbVie, and Faron Pharmaceuticals. MG reports grants from NIH/NHLBI and Agency for Healthcare Research and Quality (AHRQ), consulting fees from Endpoint, a leadership role on the American Thoracic Society (ATS) executive committee and board as well as support from ATS for meeting travel expenses, and participation on a DSMB for Regeneron. CG reports grants from NIH, CDC, Food and Drug Administration, AHRQ, Sanofi, and Syneos Health and consulting fees from Pfizer, Merck, and Sanofi. DNH reports grants from NIH/NHLBI for the ACTIV-4HT NECTAR trial and Incyte and participation as a DSMB chair for the SAFE EVICT Trial of vitamin C in COVID-19. NH reports grants from NIH, Quidel, and Sanofi and honorariums for speaking at the American Academy of Pediatrics (AAP) conference. CLH reports grants from NIH and American Lung Association (ALA) and participation as a DSMB member for iSPY COVID and Team (ANZICS). NJJ reports grants from NIH/NHLBI/NINDS and the University of Washington Royalty Research Fund and payment for expert testimony for the Washington Department of Health. AK reports grants from United Therapeutics, Gilead Sciences, and 4D Medical and a leadership role on the guidelines committee for Chest. JHK reports grants from NIH/NIAID. ASL reports grants from CDC, NIH/NIAID, and Burroughs Wellcome Fund and consulting fees from Sanofi and Roche. CJL reports grants from NIH, DoD, CDC, bioMerieux, Entegrion, Endpoint Health, and AbbVie, patents for risk stratification in sepsis and septic shock, participation on DSMBs for clinical trials unrelated to the current work, a leadership role on the executive committee for the Board of Directors of the Association for Clinical and Translational Science, and stock options in Bioscape Digita. ETM reports grants from Merck, CDC, and NIH and payment/honorariums from the Michigan Infectious Disease Society. TM reports payment/honorariums from the Society of Hospital Medicine. AM reports grants from CDC and NIAID/NIH and participation on a DSMB for the FDA. IDP reports grants from NIH, Janssen, Regeneron, and Asahi Kasei Pharma. TR reports grants from AbbVie, consulting fees from Cumberland Pharmaceuticals, and Cytovale, membership on a DSMB for Sanofi, a leadership role as immediate past president of the American Society of Parenteral and Enteral Nutrition, and stock options in Cumberland Pharmaceuticals. WHS reports receiving the primary funding for this project from the CDC, and research funding from Merck and Gilead Sciences. WBS reports grants from the NIH/NHLBI.
Figures




Update of
-
Vaccine Effectiveness of Primary Series and Booster Doses against Omicron Variant COVID-19-Associated Hospitalization in the United States.medRxiv [Preprint]. 2022 Jun 14:2022.06.09.22276228. doi: 10.1101/2022.06.09.22276228. medRxiv. 2022. Update in: BMJ. 2022 Oct 11;379:e072065. doi: 10.1136/bmj-2022-072065. PMID: 35734090 Free PMC article. Updated. Preprint.
References
-
- Lambrou AS, Shirk P, Steele MK, et al. Strain Surveillance and Emerging Variants Bioinformatic Working Group. Strain Surveillance and Emerging Variants NS3 Working Group . Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants - United States, June 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:206-11. 10.15585/mmwr.mm7106a4. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. COVID Data Tracker. 2020. https://covid.cdc.gov/covid-data-tracker (accessed 12 Aug 2022).
-
- Wilhelm A, Widera M, Grikscheit K, et al. Reduced Neutralization of SARS-CoV-2 Omicron Variant by Vaccine Sera and Monoclonal Antibodies. medRxiv 2021; 10.1101/2021.12.07.21267432. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
