Angiotensin II receptor type 1 blockade regulates Klotho expression to induce TSC2-deficient cell death
- PMID: 36220392
- PMCID: PMC9661712
- DOI: 10.1016/j.jbc.2022.102580
Angiotensin II receptor type 1 blockade regulates Klotho expression to induce TSC2-deficient cell death
Abstract
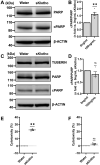
Lymphangioleiomyomatosis (LAM) is a multisystem disease occurring in women of child-bearing age manifested by uncontrolled proliferation of smooth muscle-like "LAM" cells in the lungs. LAM cells bear loss-of-function mutations in tuberous sclerosis complex (TSC) genes TSC1 and/or TSC2, causing hyperactivation of the proliferation promoting mammalian/mechanistic target of Rapamycin complex 1 pathway. Additionally, LAM-specific active renin-angiotensin system (RAS) has been identified in LAM nodules, suggesting this system potentially contributes to neoplastic properties of LAM cells; however, the role of this renin-angiotensin signaling is unclear. Here, we report that TSC2-deficient cells are sensitive to the blockade of angiotensin II receptor type 1 (Agtr1). We show that treatment of these cells with the AGTR1 inhibitor losartan or silencing of the Agtr1 gene leads to increased cell death in vitro and attenuates tumor progression in vivo. Notably, we found the effect of Agtr1 blockade is specific to TSC2-deficient cells. Mechanistically, we demonstrate that cell death induced by Agtr1 inhibition is mediated by an increased expression of Klotho. In TSC2-deficient cells, we showed overexpression of Klotho or treatment with recombinant (soluble) Klotho mirrored the cytocidal effect of angiotensin blockade. Furthermore, Klotho treatment decreased the phosphorylation of AKT, potentially leading to this cytocidal effect. Conversely, silencing of Klotho rescued TSC2-deficient cells from cell death induced by Agtr1 inhibition. Therefore, we conclude that Agtr1 and Klotho are important for TSC2-deficient cell survival. These findings further illuminate the role of the RAS in LAM and the potential of targeting Agtr1 inhibition in TSC2-deficient cells.
Keywords: Agtr1; LAM; angiotensin II; cell death; klotho; losartan; receptor; signaling; tuberin.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Smolarek T.A., Wessner L.L., McCormack F.X., Mylet J.C., Menon A.G., Henske E.P. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am. J. Hum. Genet. 1998;62:810–815. - PMC - PubMed
-
- Chan J.A., Zhang H., Roberts P.S., Jozwiak S., Wieslawa G., Lewin-Kowalik J., et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J. Neuropathol. Exp. Neurol. 2004;63:1236–1242. - PubMed
-
- El-Hashemite N., Zhang H., Henske E.P., Kwiatkowski D.J. Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet. 2003;361:1348–1349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

