Moderate hyperuricaemia ameliorated kidney damage in a low-renin model of experimental renal insufficiency
- PMID: 36220802
- PMCID: PMC10091954
- DOI: 10.1111/bcpt.13806
Moderate hyperuricaemia ameliorated kidney damage in a low-renin model of experimental renal insufficiency
Abstract
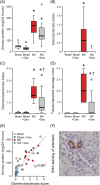
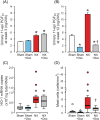
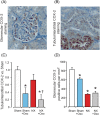
Uric acid has promoted renal fibrosis and inflammation in experimental studies, but some studies have shown nephroprotective effects due to alleviated oxidative stress. We studied the influence of experimental hyperuricaemia in surgically 5/6 nephrectomized rats. Three weeks after subtotal nephrectomy or sham operation, the rats were allocated to control diet or 2.0% oxonic acid (uricase inhibitor) diet for 9 weeks. Then blood, urine and tissue samples were taken, and renal morphology and oxidative stress were examined. Inflammation and fibrosis were evaluated using immunohistochemistry and real-time PCR (RT-PCR). Remnant kidney rats ingesting normal or oxonic acid diet presented with ~60% reduction of creatinine clearance and suppressed plasma renin activity. Oxonic acid diet increased plasma uric acid levels by >80 μmol/L. In remnant kidney rats, moderate hyperuricaemia decreased glomerulosclerosis, tubulointerstitial damage and kidney mast cell count, without influencing the fibrosis marker collagen I messenger RNA (mRNA) content. In both sham-operated and 5/6 nephrectomized rats, the mast cell product 11-epi-prostaglandin-F2α excretion to the urine and kidney tissue cyclooxygenase-2 (COX-2) levels were decreased. To conclude, hyperuricaemic remnant kidney rats displayed improved kidney morphology and reduced markers of oxidative stress and inflammation. Thus, moderately elevated plasma uric acid had beneficial effects on the kidney in this low-renin model of experimental renal insufficiency.
Keywords: experimental renal insufficiency; hyperuricaemia; kidney morphology; oxonic acid.
© 2022 The Authors. Basic & Clinical Pharmacology & Toxicology published by John Wiley & Sons Ltd on behalf of Nordic Association for the Publication of BCPT (former Nordic Pharmacological Society).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

