An ancestral interaction module promotes oligomerization in divergent mitochondrial ATP synthases
- PMID: 36220811
- PMCID: PMC9553925
- DOI: 10.1038/s41467-022-33588-z
An ancestral interaction module promotes oligomerization in divergent mitochondrial ATP synthases
Abstract
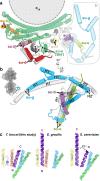
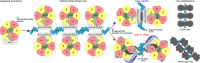
Mitochondrial ATP synthase forms stable dimers arranged into oligomeric assemblies that generate the inner-membrane curvature essential for efficient energy conversion. Here, we report cryo-EM structures of the intact ATP synthase dimer from Trypanosoma brucei in ten different rotational states. The model consists of 25 subunits, including nine lineage-specific, as well as 36 lipids. The rotary mechanism is influenced by the divergent peripheral stalk, conferring a greater conformational flexibility. Proton transfer in the lumenal half-channel occurs via a chain of five ordered water molecules. The dimerization interface is formed by subunit-g that is critical for interactions but not for the catalytic activity. Although overall dimer architecture varies among eukaryotes, we find that subunit-g together with subunit-e form an ancestral oligomerization motif, which is shared between the trypanosomal and mammalian lineages. Therefore, our data defines the subunit-g/e module as a structural component determining ATP synthase oligomeric assemblies.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
The Ancestral Shape of the Access Proton Path of Mitochondrial ATP Synthases Revealed by a Split Subunit-a.Mol Biol Evol. 2023 Jun 1;40(6):msad146. doi: 10.1093/molbev/msad146. Mol Biol Evol. 2023. PMID: 37338543 Free PMC article.
-
The peripheral stalk participates in the yeast ATP synthase dimerization independently of e and g subunits.Biochemistry. 2006 May 30;45(21):6715-23. doi: 10.1021/bi0601407. Biochemistry. 2006. PMID: 16716082
-
Horizontal membrane-intrinsic α-helices in the stator a-subunit of an F-type ATP synthase.Nature. 2015 May 14;521(7551):237-40. doi: 10.1038/nature14185. Epub 2015 Feb 23. Nature. 2015. PMID: 25707805
-
Structures and interactions of proteins involved in the coupling function of the protonmotive F(o)F(1)-ATP synthase.Curr Protein Pept Sci. 2002 Aug;3(4):451-60. doi: 10.2174/1389203023380558. Curr Protein Pept Sci. 2002. PMID: 12370007 Review.
-
Structure and Mechanisms of F-Type ATP Synthases.Annu Rev Biochem. 2019 Jun 20;88:515-549. doi: 10.1146/annurev-biochem-013118-110903. Epub 2019 Mar 22. Annu Rev Biochem. 2019. PMID: 30901262 Review.
Cited by
-
The mystery of massive mitochondrial complexes: the apicomplexan respiratory chain.Trends Parasitol. 2022 Dec;38(12):1041-1052. doi: 10.1016/j.pt.2022.09.008. Epub 2022 Oct 24. Trends Parasitol. 2022. PMID: 36302692 Free PMC article. Review.
-
Functional and biochemical characterization of the Toxoplasma gondii succinate dehydrogenase complex.PLoS Pathog. 2023 Dec 11;19(12):e1011867. doi: 10.1371/journal.ppat.1011867. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38079448 Free PMC article.
-
The consequence of ATP synthase dimer angle on mitochondrial morphology studied by cryo-electron tomography.Biochem J. 2024 Jan 2;481(3):161-75. doi: 10.1042/BCJ20230450. Online ahead of print. Biochem J. 2024. PMID: 38164968 Free PMC article.
-
Characterization of a highly diverged mitochondrial ATP synthase Fo subunit in Trypanosoma brucei.J Biol Chem. 2022 Apr;298(4):101829. doi: 10.1016/j.jbc.2022.101829. Epub 2022 Mar 12. J Biol Chem. 2022. PMID: 35293314 Free PMC article.
-
The Ancestral Shape of the Access Proton Path of Mitochondrial ATP Synthases Revealed by a Split Subunit-a.Mol Biol Evol. 2023 Jun 1;40(6):msad146. doi: 10.1093/molbev/msad146. Mol Biol Evol. 2023. PMID: 37338543 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

