Treatment of cardiac fibrosis: from neuro-hormonal inhibitors to CAR-T cell therapy
- PMID: 36221014
- PMCID: PMC9553301
- DOI: 10.1007/s10741-022-10279-x
Treatment of cardiac fibrosis: from neuro-hormonal inhibitors to CAR-T cell therapy
Abstract
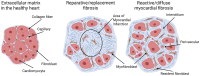
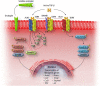
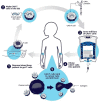
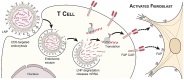
Cardiac fibrosis is characterized by the deposition of extracellular matrix proteins in the spaces between cardiomyocytes following both acute and chronic tissue damage events, resulting in the remodeling and stiffening of heart tissue. Fibrosis plays an important role in the pathogenesis of many cardiovascular disorders, including heart failure and myocardial infarction. Several studies have identified fibroblasts, which are induced to differentiate into myofibroblasts in response to various types of damage, as the most important cell types involved in the fibrotic process. Some drugs, such as inhibitors of the renin-angiotensin-aldosterone system, have been shown to be effective in reducing cardiac fibrosis. There are currently no drugs with primarily anti-fibrotic action approved for clinical use, as well as the evidence of a clinical efficacy of these drugs is extremely limited, despite the numerous encouraging results from experimental studies. A new approach is represented by the use of CAR-T cells engineered in vivo using lipid nanoparticles containing mRNA coding for a receptor directed against the FAP protein, expressed by cardiac myofibroblasts. This strategy has proved to be safe and effective in reducing myocardial fibrosis and improving cardiac function in mouse models of cardiac fibrosis. Clinical studies are required to test this novel approach in humans.
Keywords: Anti-fibrotic therapies; CAR-T cells; Fibrosis; Heart failure; Myocardium.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
[Treatment of cardiac fibrosis: from neurohormonal antagonists to CAR-T cells].G Ital Cardiol (Rome). 2023 Jul;24(7):508-520. doi: 10.1714/4060.40430. G Ital Cardiol (Rome). 2023. PMID: 37392116 Italian.
-
Targeting cardiac fibrosis with Chimeric Antigen Receptor-Engineered Cells.Mol Cell Biochem. 2025 Apr;480(4):2103-2116. doi: 10.1007/s11010-024-05134-6. Epub 2024 Oct 26. Mol Cell Biochem. 2025. PMID: 39460827 Review.
-
CAR-Macrophage Therapy Alleviates Myocardial Ischemia-Reperfusion Injury.Circ Res. 2024 Dec 6;135(12):1161-1174. doi: 10.1161/CIRCRESAHA.124.325212. Epub 2024 Oct 28. Circ Res. 2024. PMID: 39465245
-
Nuclear Receptor Nur77 Controls Cardiac Fibrosis through Distinct Actions on Fibroblasts and Cardiomyocytes.Int J Mol Sci. 2021 Feb 5;22(4):1600. doi: 10.3390/ijms22041600. Int J Mol Sci. 2021. PMID: 33562500 Free PMC article.
-
Persistent phenotypic shift in cardiac fibroblasts: impact of transient renin angiotensin system inhibition.J Mol Cell Cardiol. 2016 Apr;93:125-32. doi: 10.1016/j.yjmcc.2015.11.027. Epub 2015 Nov 27. J Mol Cell Cardiol. 2016. PMID: 26631495 Review.
Cited by
-
Ferroptosis in diabetic cardiomyopathy: Advances in cardiac fibroblast-cardiomyocyte interactions.Heliyon. 2024 Jul 28;10(15):e35219. doi: 10.1016/j.heliyon.2024.e35219. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39165946 Free PMC article. Review.
-
Post-myocardial infarction fibrosis: Pathophysiology, examination, and intervention.Front Pharmacol. 2023 Mar 28;14:1070973. doi: 10.3389/fphar.2023.1070973. eCollection 2023. Front Pharmacol. 2023. PMID: 37056987 Free PMC article. Review.
-
Statins and thyroid eye disease (TED): a systematic review.Endocrine. 2024 Jul;85(1):11-17. doi: 10.1007/s12020-023-03680-5. Epub 2024 Jan 9. Endocrine. 2024. PMID: 38194219
-
Inhibitory effect of microRNA-21 on pathways and mechanisms involved in cardiac fibrosis development.Ther Adv Cardiovasc Dis. 2024 Jan-Dec;18:17539447241253134. doi: 10.1177/17539447241253134. Ther Adv Cardiovasc Dis. 2024. PMID: 38819836 Free PMC article. Review.
-
The Macrophage-Fibroblast Dipole in the Context of Cardiac Repair and Fibrosis.Biomolecules. 2024 Nov 4;14(11):1403. doi: 10.3390/biom14111403. Biomolecules. 2024. PMID: 39595580 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

