Pre-referral intranasal artesunate powder for cerebral malaria: a proof-of-concept study
- PMID: 36221071
- PMCID: PMC9555123
- DOI: 10.1186/s12936-022-04309-0
Pre-referral intranasal artesunate powder for cerebral malaria: a proof-of-concept study
Abstract
Background: Malaria still kills young children in rural endemic areas because early treatment is not available. Thus, the World Health Organization recommends the administration of artesunate suppositories as pre-referral treatment before transportation to the hospital in case of severe symptoms with an unavailable parenteral and oral treatment. However, negative cultural perception of the rectal route, and limited access to artesunate suppositories, could limit the use of artesunate suppositories. There is, therefore, a need for an alternative route for malaria pre-referral treatment. The aim of this study was to assess the potential of intranasal route for malaria pre-referral treatment.
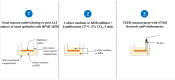
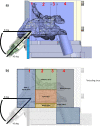
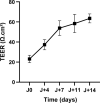
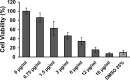
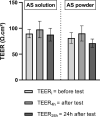
Methods: The permeability of artesunate through human nasal mucosa was tested in vitro. The Transepithelial Electrical Resistance (TEER) of the nasal mucosa was followed during the permeation tests. Beside, regional deposition of artesunate powder was assessed with an unidose drug delivery device in each nostril of a nasal cast. Artesunate quantification was performed using Liquid Chromatography coupled to tandem Mass Spectrometry.
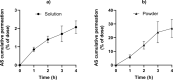
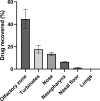
Results: The experimental model of human nasal mucosa was successfully implemented. Using this model, artesunate powder showed a much better passage rate through human nasal mucosa than solution (26.8 ± 6.6% versus 2.1 ± 0.3%). More than half (62.3%) of the artesunate dose sprayed in the nostrils of the nasal cast was recovered in the olfactory areas (44.7 ± 8.6%) and turbinates (17.6 ± 3.3%) allowing nose-to-brain and systemic drug diffusion, respectively.
Conclusion: Artesunate powder showed a good permeation efficiency on human nasal mucosa. Moreover it can be efficiently sprayed in the nostrils using unidose device to reach the olfactory area leading to a fast nose-to-brain delivery as well as a systemic effect. Taken together, those results are part of the proof-of-concept for the use of intranasal artesunate as a malaria pre-referral treatment.
Keywords: Artesunate; Nasal cast; Nasal mucosa; Nose-to-brain delivery; Pre-referral treatment; Severe malaria.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- WHO. World malaria report 2021 [Internet]. Geneva: World Health Organization; 2021 [cited 2021 Dec 9]. Available from: https://apps.who.int/iris/handle/10665/350147.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

