Exosomes: mediators regulating the phenotypic transition of vascular smooth muscle cells in atherosclerosis
- PMID: 36221105
- PMCID: PMC9555104
- DOI: 10.1186/s12964-022-00949-6
Exosomes: mediators regulating the phenotypic transition of vascular smooth muscle cells in atherosclerosis
Abstract
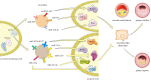
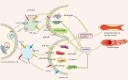
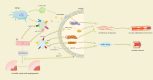
Cardiovascular disease is one of the leading causes of human mortality worldwide, mainly due to atherosclerosis (AS), and the phenotypic transition of vascular smooth muscle cells (VSMCs) is a key event in the development of AS. Exosomes contain a variety of specific nucleic acids and proteins that mediate intercellular communication. The role of exosomes in AS has attracted attention. This review uses the VSMC phenotypic transition in AS as the entry point, introduces the effect of exosomes on AS from different perspectives, and discusses the status quo, deficiencies, and potential future directions in this field to provide new ideas for clinical research and treatment of AS. Video Abstract.
Keywords: Atherosclerosis; Exosomes; Phenotypic transition; Vascular smooth muscle cells.
© 2022. The Author(s).
Conflict of interest statement
None declared.
Figures
Similar articles
-
Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation.Theranostics. 2019 Sep 21;9(23):6901-6919. doi: 10.7150/thno.37357. eCollection 2019. Theranostics. 2019. PMID: 31660076 Free PMC article.
-
Exosomes: Multifaceted Messengers in Atherosclerosis.Curr Atheroscler Rep. 2020 Aug 9;22(10):57. doi: 10.1007/s11883-020-00871-7. Curr Atheroscler Rep. 2020. PMID: 32772195 Review.
-
Exosomal LINC01005 derived from oxidized low-density lipoprotein-treated endothelial cells regulates vascular smooth muscle cell phenotypic switch.Biofactors. 2020 Sep;46(5):743-753. doi: 10.1002/biof.1665. Epub 2020 Jul 14. Biofactors. 2020. PMID: 32663367
-
Activation of CD137 signaling promotes neointimal formation by attenuating TET2 and transferrring from endothelial cell-derived exosomes to vascular smooth muscle cells.Biomed Pharmacother. 2020 Jan;121:109593. doi: 10.1016/j.biopha.2019.109593. Epub 2019 Nov 19. Biomed Pharmacother. 2020. PMID: 31766102
-
Emerging role of exosomes in arterial and renal calcification.Hum Exp Toxicol. 2021 Sep;40(9):1385-1402. doi: 10.1177/09603271211001122. Epub 2021 Mar 19. Hum Exp Toxicol. 2021. PMID: 33739177 Review.
Cited by
-
Removing the stumbling block of exosome applications in clinical and translational medicine: expand production and improve accuracy.Stem Cell Res Ther. 2023 Apr 1;14(1):57. doi: 10.1186/s13287-023-03288-6. Stem Cell Res Ther. 2023. PMID: 37005658 Free PMC article. Review.
-
Exosome miR-199a-5p modulated vascular remodeling and inflammatory infiltration of Takayasu's arteritis.Arthritis Res Ther. 2025 Jan 20;27(1):11. doi: 10.1186/s13075-025-03475-1. Arthritis Res Ther. 2025. PMID: 39833857 Free PMC article.
-
Enhanced secretion of promyogenic exosomes by quiescent muscle cells.Front Cell Dev Biol. 2024 Jul 23;12:1381357. doi: 10.3389/fcell.2024.1381357. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39108837 Free PMC article.
-
The Physiological Functions and Therapeutic Potential of Hypoxia-Inducible Factor-1α in Vascular Calcification.Biomolecules. 2024 Dec 12;14(12):1592. doi: 10.3390/biom14121592. Biomolecules. 2024. PMID: 39766299 Free PMC article. Review.
-
The Role of Macrophages in Atherosclerosis: Participants and Therapists.Cardiovasc Drugs Ther. 2025 Apr;39(2):459-472. doi: 10.1007/s10557-023-07513-5. Epub 2023 Oct 21. Cardiovasc Drugs Ther. 2025. PMID: 37864633 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

