The role of skin inflammation, barrier dysfunction, and oral tolerance in skin sensitization to gluten-derived hydrolysates in a rat model
- PMID: 36221232
- PMCID: PMC10091953
- DOI: 10.1111/cod.14233
The role of skin inflammation, barrier dysfunction, and oral tolerance in skin sensitization to gluten-derived hydrolysates in a rat model
Abstract
Background: Adverse reactions to wheat-containing skin care products have been linked to food allergy development.
Objectives: To determine the role of skin barrier dysfunction and inflammation in sensitization to gluten-derived hydrolysates via the skin in Brown Norway rats with and without oral tolerance to wheat.
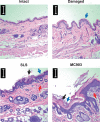
Methods: Skin barrier defect was induced by mechanical disruption, and skin inflammation was induced by topical application of SLS or MC903. Unmodified, enzyme hydrolyzed, or acid hydrolyzed gluten products were applied to the skin three times per week for 5 weeks. Subsequently, rats were orally gavaged with unmodified gluten.
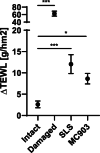
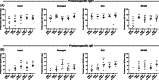
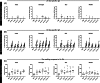
Results: Wheat-naïve rats were readily sensitized to gluten hydrolysates via the skin. Skin barrier defect and skin inflammation had little effect on the skin sensitization and hydrolysate-specific IgE levels. Oral administration of unmodified gluten promoted the production of unmodified gluten-specific IgE in rats sensitized via the skin. Sensitization through intact skin, disrupted skin barrier, or inflamed skin was unable to break tolerance to unmodified gluten in rats on a wheat-containing diet.
Conclusions: Mechanical skin barrier disruption and skin inflammation play a limited role in experimental skin sensitization to gluten-derived hydrolysates.
Keywords: animal model; food allergy; gluten; oral tolerance; skin barrier dysfunction; skin inflammation; skin sensitization; wheat.
© 2022 The Authors. Contact Dermatitis published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest. The funding agencies and the providers of gluten‐derived products played no role in study design, data acquisition, data analysis, interpretation, manuscript preparation, or the decision to publish.
Figures


Similar articles
-
Acid Hydrolysis of Gluten Enhances the Skin Sensitizing Potential and Drives Diversification of IgE Reactivity to Unmodified Gluten Proteins.Mol Nutr Food Res. 2021 Dec;65(23):e2100416. doi: 10.1002/mnfr.202100416. Epub 2021 Oct 27. Mol Nutr Food Res. 2021. PMID: 34636481
-
Gluten tolerance prevents oral sensitization with enzymatic or acid hydrolyzed gluten: A study in Brown Norway rats.PLoS One. 2020 Apr 6;15(4):e0231139. doi: 10.1371/journal.pone.0231139. eCollection 2020. PLoS One. 2020. PMID: 32251478 Free PMC article.
-
Acid hydrolysis of wheat gluten induces formation of new epitopes but does not enhance sensitizing capacity by the oral route: a study in "gluten free" Brown Norway rats.PLoS One. 2014 Sep 10;9(9):e107137. doi: 10.1371/journal.pone.0107137. eCollection 2014. PLoS One. 2014. PMID: 25207551 Free PMC article.
-
Allergic reactions to hydrolysed wheat proteins: clinical aspects and molecular structures of the allergens involved.Crit Rev Food Sci Nutr. 2020;60(1):147-156. doi: 10.1080/10408398.2018.1516622. Epub 2018 Nov 21. Crit Rev Food Sci Nutr. 2020. PMID: 30463417 Review.
-
Skin barrier dysfunction and systemic sensitization to allergens through the skin.Curr Drug Targets Inflamm Allergy. 2005 Oct;4(5):531-41. doi: 10.2174/156801005774322199. Curr Drug Targets Inflamm Allergy. 2005. PMID: 16248822 Review.
References
-
- Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141:41‐58. - PubMed
-
- Lack G, Fox D, Northstone K, Golding J, Avon Longitudinal Study of Parents and Children Study Team . Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977‐985. - PubMed
-
- Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35:757‐766. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

