A Sterically Tuned Directing Auxiliary Promotes Catalytic 1,2-Carbofluorination of Alkenyl Carbonyl Compounds
- PMID: 36221812
- PMCID: PMC9851970
- DOI: 10.1002/anie.202214153
A Sterically Tuned Directing Auxiliary Promotes Catalytic 1,2-Carbofluorination of Alkenyl Carbonyl Compounds
Abstract
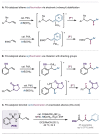
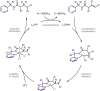
The site-selective palladium-catalyzed three-component coupling of unactivated alkenyl carbonyl compounds, aryl- or alkenylboronic acids, and N-fluorobenzenesulfonimide is described herein. Tuning of the steric environment on the bidentate directing auxiliary enhances regioselectivity and facilitates challenging C(sp3 )-F reductive elimination from a PdIV intermediate to afford 1,2-carbofluorination products in moderate to good yields.
Keywords: Alkene Functionalization; Cross-Coupling; Directing Group; Fluorination; Palladium.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Recent advances in efficient and selective synthesis of di-, tri-, and tetrasubstituted alkenes via Pd-catalyzed alkenylation-carbonyl olefination synergy.Acc Chem Res. 2008 Nov 18;41(11):1474-85. doi: 10.1021/ar800038e. Acc Chem Res. 2008. PMID: 18783256
-
Regio- and Stereoselective 1,2-Oxyhalogenation of Non-Conjugated Alkynes via Directed Nucleopalladation: Catalytic Access to Tetrasubstituted Alkenes.Angew Chem Int Ed Engl. 2022 Oct 24;61(43):e202209099. doi: 10.1002/anie.202209099. Epub 2022 Sep 29. Angew Chem Int Ed Engl. 2022. PMID: 36082442 Free PMC article.
-
Directed, Palladium(II)-Catalyzed Intermolecular Aminohydroxylation of Alkenes Using a Mild Oxidation System.Org Lett. 2018 Jul 6;20(13):3853-3857. doi: 10.1021/acs.orglett.8b01440. Epub 2018 Jun 11. Org Lett. 2018. PMID: 29888604 Free PMC article.
-
Enantioselective Dicarbofunctionalization of Unactivated Alkenes by Palladium-Catalyzed Tandem Heck/Suzuki Coupling Reaction.Angew Chem Int Ed Engl. 2019 Oct 7;58(41):14653-14659. doi: 10.1002/anie.201907840. Epub 2019 Sep 5. Angew Chem Int Ed Engl. 2019. PMID: 31420928 Review.
-
Realization of Anti-Markovnikov Selectivity in Pd-Catalyzed Oxidative Acetalization and Wacker-Type Oxidation of Terminal Alkenes.Chem Rec. 2021 Dec;21(12):3458-3469. doi: 10.1002/tcr.202100090. Epub 2021 May 21. Chem Rec. 2021. PMID: 34021681 Review.
Cited by
-
Divergent Synthesis of β-Fluoroamides via Silver-Catalyzed Oxidative Deconstruction of Cyclopropanone Hemiaminals.Org Lett. 2023 Jul 21;25(28):5389-5394. doi: 10.1021/acs.orglett.3c01992. Epub 2023 Jul 6. Org Lett. 2023. PMID: 37413978 Free PMC article.
-
A Catalytic Three-Component Aminofluorination of Unactivated Alkenes with Electron-Rich Amino Sources.Adv Sci (Weinh). 2024 Mar;11(12):e2305006. doi: 10.1002/advs.202305006. Epub 2024 Jan 16. Adv Sci (Weinh). 2024. PMID: 38226424 Free PMC article.
-
One-Pot Formal Carboradiofluorination of Alkenes: A Toolkit for Positron Emission Tomography Imaging Probe Development.J Am Chem Soc. 2023 Sep 6;145(35):19265-19273. doi: 10.1021/jacs.3c04548. Epub 2023 Aug 25. J Am Chem Soc. 2023. PMID: 37625118 Free PMC article.
References
-
- None
-
- Gillis E. P., Eastman K. J., Hill M. D., Donnelly D. J., Meanwell N. A., J. Med. Chem. 2015, 58, 8315–8359; - PubMed
-
- Purser S., Moore P. R., Swallow S., Gouverneur V., Chem. Soc. Rev. 2008, 37, 320–330; - PubMed
-
- Wolstenhulme J. R., Gouverneur V., Acc. Chem. Res. 2014, 47, 3560–3570. - PubMed
-
- None
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

