Suramin enhances chondrogenic properties by regulating the p67phox/PI3K/AKT/SOX9 signalling pathway
- PMID: 36222195
- PMCID: PMC9582866
- DOI: 10.1302/2046-3758.1110.BJR-2022-0013.R2
Suramin enhances chondrogenic properties by regulating the p67phox/PI3K/AKT/SOX9 signalling pathway
Abstract
Aims: Autologous chondrocyte implantation (ACI) is a promising treatment for articular cartilage degeneration and injury; however, it requires a large number of human hyaline chondrocytes, which often undergo dedifferentiation during in vitro expansion. This study aimed to investigate the effect of suramin on chondrocyte differentiation and its underlying mechanism.
Methods: Porcine chondrocytes were treated with vehicle or various doses of suramin. The expression of collagen, type II, alpha 1 (COL2A1), aggrecan (ACAN); COL1A1; COL10A1; SRY-box transcription factor 9 (SOX9); nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX); interleukin (IL)-1β; tumour necrosis factor alpha (TNFα); IL-8; and matrix metallopeptidase 13 (MMP-13) in chondrocytes at both messenger RNA (mRNA) and protein levels was determined by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and western blot. In addition, the supplementation of suramin to redifferentiation medium for the culture of expanded chondrocytes in 3D pellets was evaluated. Glycosaminoglycan (GAG) and collagen production were evaluated by biochemical analyses and immunofluorescence, as well as by immunohistochemistry. The expression of reactive oxygen species (ROS) and NOX activity were assessed by luciferase reporter gene assay, immunofluorescence analysis, and flow cytometry. Mutagenesis analysis, Alcian blue staining, reverse transcriptase polymerase chain reaction (RT-PCR), and western blot assay were used to determine whether p67phox was involved in suramin-enhanced chondrocyte phenotype maintenance.
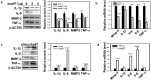
Results: Suramin enhanced the COL2A1 and ACAN expression and lowered COL1A1 synthesis. Also, in 3D pellet culture GAG and COL2A1 production was significantly higher in pellets consisting of chondrocytes expanded with suramin compared to controls. Surprisingly, suramin also increased ROS generation, which is largely caused by enhanced NOX (p67phox) activity and membrane translocation. Overexpression of p67phox but not p67phoxAD (deleting amino acid (a.a) 199 to 212) mutant, which does not support ROS production in chondrocytes, significantly enhanced chondrocyte phenotype maintenance, SOX9 expression, and AKT (S473) phosphorylation. Knockdown of p67phox with its specific short hairpin (sh) RNA (shRNA) abolished the suramin-induced effects. Moreover, when these cells were treated with the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) inhibitor LY294002 or shRNA of AKT1, p67phox-induced COL2A1 and ACAN expression was significantly inhibited.
Conclusion: Suramin could redifferentiate dedifferentiated chondrocytes dependent on p67phox activation, which is mediated by the PI3K/AKT/SOX9 signalling pathway.Cite this article: Bone Joint Res 2022;11(10):693-708.
Keywords: Alcian blue; Chondrocyte redifferentiation; Col2a1; NADPH oxidase; RNA; Reactive oxygen species; Suramin; chondrocytes; collagen; mRNA; p67phox; phosphate; reverse transcriptase-polymerase chain reaction; staining; western blot.
Figures








References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

