A prospective clinical study on the mechanisms underlying critical illness myopathy-A time-course approach
- PMID: 36222215
- PMCID: PMC9745499
- DOI: 10.1002/jcsm.13104
A prospective clinical study on the mechanisms underlying critical illness myopathy-A time-course approach
Abstract
Background: Critical illness myopathy (CIM) is a consequence of modern critical care resulting in general muscle wasting and paralyses of all limb and trunk muscles, resulting in prolonged weaning from the ventilator, intensive care unit (ICU) treatment and rehabilitation. CIM is associated with severe morbidity/mortality and significant negative socioeconomic consequences, which has become increasingly evident during the current COVID-19 pandemic, but underlying mechanisms remain elusive.
Methods: Ten neuro-ICU patients exposed to long-term controlled mechanical ventilation were followed with repeated muscle biopsies, electrophysiology and plasma collection three times per week for up to 12 days. Single muscle fibre contractile recordings were conducted on the first and final biopsy, and a multiomics approach was taken to analyse gene and protein expression in muscle and plasma at all collection time points.
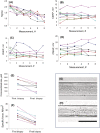
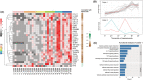
Results: (i) A progressive preferential myosin loss, the hallmark of CIM, was observed in all neuro-ICU patients during the observation period (myosin:actin ratio decreased from 2.0 in the first to 0.9 in the final biopsy, P < 0.001). The myosin loss was coupled to a general transcriptional downregulation of myofibrillar proteins (P < 0.05; absolute fold change >2) and activation of protein degradation pathways (false discovery rate [FDR] <0.1), resulting in significant muscle fibre atrophy and loss in force generation capacity, which declined >65% during the 12 day observation period (muscle fibre cross-sectional area [CSA] and maximum single muscle fibre force normalized to CSA [specific force] declined 30% [P < 0.007] and 50% [P < 0.0001], respectively). (ii) Membrane excitability was not affected as indicated by the maintained compound muscle action potential amplitude upon supramaximal stimulation of upper and lower extremity motor nerves. (iii) Analyses of plasma revealed early activation of inflammatory and proinflammatory pathways (FDR < 0.1), as well as a redistribution of zinc ions from plasma.
Conclusions: The mechanical ventilation-induced lung injury with release of cytokines/chemokines and the complete mechanical silencing uniquely observed in immobilized ICU patients affecting skeletal muscle gene/protein expression are forwarded as the dominant factors triggering CIM.
Keywords: critical illness myopathy; mechanical ventilation; membrane exitability; muscle paresis; myosin loss.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors declare no financial or nonfinancial competing interests. Written and verbal informed consent was obtained from a close relative, and the study was approved by the ethics committee at the Karolinska Hospital (Dnr 2016/242‐31/2). The study conforms to the ethical guidelines of the Journal of Cachexia, Sarcopenia and Muscle.
Figures



References
-
- Zilberberg MD, Luippold RS, Sulsky S, Shorr AF. Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med 2008;36:724–730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

