Redesign of Rifamycin Antibiotics to Overcome ADP-Ribosylation-Mediated Resistance
- PMID: 36222275
- PMCID: PMC9633546
- DOI: 10.1002/anie.202211498
Redesign of Rifamycin Antibiotics to Overcome ADP-Ribosylation-Mediated Resistance
Abstract
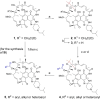
Rifamycin antibiotics are a valuable class of antimicrobials for treating infections by mycobacteria and other persistent bacteria owing to their potent bactericidal activity against replicating and non-replicating pathogens. However, the clinical utility of rifamycins against Mycobacterium abscessus is seriously compromised by a novel resistance mechanism, namely, rifamycin inactivation by ADP-ribosylation. Using a structure-based approach, we rationally redesign rifamycins through strategic modification of the ansa-chain to block ADP-ribosylation while preserving on-target activity. Validated by a combination of biochemical, structural, and microbiological studies, the most potent analogs overcome ADP-ribosylation, restored their intrinsic low nanomolar activity and demonstrated significant in vivo antibacterial efficacy. Further optimization by tuning drug disposition properties afforded a preclinical candidate with remarkable potency and an outstanding pharmacokinetic profile.
Keywords: Antibiotic Resistance; Antibiotics; Drug Design; Mycobacterium Abscessus; Rifamycin.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Murray C. J. L., Ikuta K. S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., Han C., Bisignano C., Rao P., Wool E., Johnson S. C., Browne A. J., Chipeta M. G., Fell F., Hackett S., Haines-Woodhouse G., Kashef Hamadani B. H., Kumaran E. A. P., McManigal B., Agarwal R., Akech S., Albertson S., Amuasi J., Andrews J., Aravkin A., Ashley E., Bailey F., Baker S., Basnyat B., Bekker A., Bender R., Bethou A., Bielicki J., Boonkasidecha S., Bukosia J., Carvalheiro C., Castañeda-Orjuela C., Chansamouth V., Chaurasia S., Chiurchiù S., Chowdhury F., Cook A. J., Cooper B., Cressey T. R., Criollo-Mora E., Cunningham M., Darboe S., Day N. P. J., De Luca M., Dokova K., Dramowski A., Dunachie S. J., Eckmanns T., Eibach D., Emami A., Feasey N., Fisher-Pearson N., Forrest K., Garrett D., Gastmeier P., Giref A. Z., Greer R. C., Gupta V., Haller S., Haselbeck A., Hay S. I., Holm M., Hopkins S., Iregbu K. C., Jacobs J., Jarovsky D., Javanmardi F., Khorana M., Kissoon N., Kobeissi E., Kostyanev T., Krapp F., Krumkamp R., Kumar A., Kyu H. H., Lim C., Limmathurotsakul D., Loftus M. J., Lunn M., Ma J., Mturi N., Munera-Huertas T., Musicha P., Mussi-Pinhata M. M., Nakamura T., Nanavati R., Nangia S., Newton P., Ngoun C., Novotney A., Nwakanma D., Obiero C. W., Olivas-Martinez A., Olliaro P., Ooko E., Ortiz-Brizuela E., Peleg A. Y., Perrone C., Plakkal N., Ponce-de-Leon A., Raad M., Ramdin T., Riddell A., Roberts T., Robotham J. V., Roca A., Rudd K. E., Russell N., Schnall J., Scott J. A. G., Shivamallappa M., Sifuentes-Osornio J., Steenkeste N., Stewardson A. J., Stoeva T., Tasak N., Thaiprakong A., Thwaites G., Turner C., Turner P., van Doorn H. R., Velaphi S., Vongpradith A., Vu H., Walsh T., Waner S., Wangrangsimakul T., Wozniak T., Zheng P., Sartorius B., Lopez A. D., Stergachis A., Moore C., Dolecek C., Naghavi M., Lancet 2022, 399, 629–655. - PMC - PubMed
-
- Abraham E. P., Chain E., Nature 1940, 146, 837–837.
-
- Wright G. D., Adv. Drug Delivery Rev. 2005, 57, 1451–1470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

