Efficacy of APX2039 in a Rabbit Model of Cryptococcal Meningitis
- PMID: 36222509
- PMCID: PMC9765414
- DOI: 10.1128/mbio.02347-22
Efficacy of APX2039 in a Rabbit Model of Cryptococcal Meningitis
Abstract
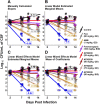
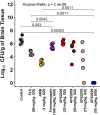
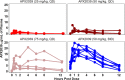
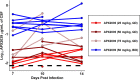
Cryptococcal Meningitis (CM) is uniformly fatal if not treated, and treatment options are limited. We previously reported on the activity of APX2096, the prodrug of the novel Gwt1 inhibitor APX2039, in a mouse model of CM. Here, we investigated the efficacy of APX2039 in mouse and rabbit models of CM. In the mouse model, the controls had a mean lung fungal burden of 5.95 log10 CFU/g, whereas those in the fluconazole-, amphotericin B-, and APX2039-treated mice were 3.56, 4.59, and 1.50 log10 CFU/g, respectively. In the brain, the control mean fungal burden was 7.97 log10 CFU/g, while the burdens were 4.64, 7.16, and 1.44 log10 CFU/g for treatment with fluconazole, amphotericin B, and APX2039, respectively. In the rabbit model of CM, the oral administration of APX2039 at 50 mg/kg of body weight twice a day (BID) resulted in a rapid decrease in the cerebrospinal fluid (CSF) fungal burden, and the burden was below the limit of detection by day 10 postinfection. The effective fungicidal activity (EFA) was -0.66 log10 CFU/mL/day, decreasing from an average of 4.75 log10 CFU/mL to 0 CFU/mL, over 8 days of therapy, comparing favorably with good clinical outcomes in humans associated with reductions of the CSF fungal burden of -0.4 log10 CFU/mL/day, and, remarkably, 2-fold the EFA of amphotericin B deoxycholate in this model (-0.33 log10 CFU/mL/day). A total drug exposure of the area under the concentration-time curve from 0 to 24 h (AUC0-24) of 25 to 50 mg · h/L of APX2039 resulted in near-maximal antifungal activity. These data support the further preclinical and clinical evaluation of APX2039 as a new oral fungicidal monotherapy for the treatment of CM. IMPORTANCE Cryptococcal meningitis (CM) is a fungal disease with significant global morbidity and mortality. The gepix Gwt1 inhibitors are a new class of antifungal drugs. Here, we demonstrated the efficacy of APX2039, the second member of the gepix class, in rabbit and mouse models of cryptococcal meningitis. We also analyzed the drug levels in the blood and cerebrospinal fluid in the highly predictive rabbit model and built a mathematical model to describe the behavior of the drug with respect to the elimination of the fungal pathogen. We demonstrated that the oral administration of APX2039 resulted in a rapid decrease in the CSF fungal burden, with an effective fungicidal activity of -0.66 log10 CFU/mL/day, comparing favorably with good clinical outcomes in humans associated with reductions of -0.4 log10 CFU/mL/day. The drug APX2039 had good penetration of the central nervous system and is an excellent candidate for future clinical testing in humans for the treatment of CM.
Keywords: APX001; APX2039; Cryptococcus; Gwt1; antifungal; fosmanogepix; gepix; manogepix; meningitis.
Conflict of interest statement
The authors declare a conflict of interest. J.R.P. has received research support from and served on advisory boards for Astellas, Pfizer, Merck, Appili, Amplyx, Matinas, Scynexis, and Minnetronix. C.D.G. was paid by projects supported by Appili, Astellas, Amplyx, Interventional Analgesix, and Minnetronix. W.H. holds or has recently held research grants with UKRI, F2G, Spero Therapeutics, Antabio, Pfizer, Bugworks, Phico Therapeutics, BioVersys, GARDP and NAEJA-RGM. He is (or has recently been) a consultant for Appili Therapeutics, F2G, Spero Therapeutics, NAEJA-RGM, Centauri, Pfizer, Phico Therapeutics and VenatoRx. He is a member of the Specialist Advisory Committee for GARDP and the Specialty National Co-lead for Infectious Diseases for the National Institute of Health Research (NIHR). Q.S. was previously an employee of Amplyx, a wholly owned subsidiary of Pfizer, and is now an employee of Pfizer. K.J.S. was previously an employee of Amplyx, and is currently a consultant for Pfizer. All other authors have no conflicts.
Figures


Similar articles
-
In Vitro and In Vivo Evaluation of APX001A/APX001 and Other Gwt1 Inhibitors against Cryptococcus.Antimicrob Agents Chemother. 2018 Jul 27;62(8):e00523-18. doi: 10.1128/AAC.00523-18. Print 2018 Aug. Antimicrob Agents Chemother. 2018. PMID: 29891599 Free PMC article.
-
Short-course amphotericin B in addition to sertraline and fluconazole for treatment of HIV-associated cryptococcal meningitis in rural Tanzania.Mycoses. 2019 Dec;62(12):1127-1132. doi: 10.1111/myc.12995. Epub 2019 Oct 21. Mycoses. 2019. PMID: 31461550 Free PMC article. Clinical Trial.
-
Cerebrospinal Fluid Early Fungicidal Activity as a Surrogate Endpoint for Cryptococcal Meningitis Survival in Clinical Trials.Clin Infect Dis. 2020 Oct 23;71(7):e45-e49. doi: 10.1093/cid/ciaa016. Clin Infect Dis. 2020. PMID: 31912875 Free PMC article.
-
Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections.Drugs. 2009;69(3):361-92. doi: 10.2165/00003495-200969030-00010. Drugs. 2009. PMID: 19275278 Review.
-
Leave no one behind: response to new evidence and guidelines for the management of cryptococcal meningitis in low-income and middle-income countries.Lancet Infect Dis. 2019 Apr;19(4):e143-e147. doi: 10.1016/S1473-3099(18)30493-6. Epub 2018 Oct 18. Lancet Infect Dis. 2019. PMID: 30344084 Review.
Cited by
-
Pharmacodynamics of ATI-2307 in a rabbit model of cryptococcal meningoencephalitis.Antimicrob Agents Chemother. 2023 Oct 18;67(10):e0081823. doi: 10.1128/aac.00818-23. Epub 2023 Sep 20. Antimicrob Agents Chemother. 2023. PMID: 37728934 Free PMC article.
-
Preclinical Models for Cryptococcosis of the CNS and Their Characterization Using In Vivo Imaging Techniques.J Fungi (Basel). 2024 Feb 12;10(2):146. doi: 10.3390/jof10020146. J Fungi (Basel). 2024. PMID: 38392818 Free PMC article. Review.
-
The Rabbit Model of Cryptococcal Meningitis.Methods Mol Biol. 2024;2775:13-27. doi: 10.1007/978-1-0716-3722-7_2. Methods Mol Biol. 2024. PMID: 38758308
-
Induction Treatment for HIV-Associated Cryptococcal Meningitis: Where Have We Been and Where Are We Going?Microorganisms. 2025 Apr 8;13(4):847. doi: 10.3390/microorganisms13040847. Microorganisms. 2025. PMID: 40284683 Free PMC article. Review.
-
In vitro manogepix susceptibility testing of South African Emergomyces africanus, Emergomyces pasteurianus, and Blastomyces emzantsi clinical isolates.Antimicrob Agents Chemother. 2023 Dec 14;67(12):e0110423. doi: 10.1128/aac.01104-23. Epub 2023 Nov 16. Antimicrob Agents Chemother. 2023. PMID: 37971237 Free PMC article.
References
-
- Anonymous. 2018. WHO guidelines approved by the Guidelines Review Committee, guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization, Geneva, Switzerland. - PubMed
-
- Jarvis JN, Lawrence DS, Meya DB, Kagimu E, Kasibante J, Mpoza E, Rutakingirwa MK, Ssebambulidde K, Tugume L, Rhein J, Boulware DR, Mwandumba HC, Moyo M, Mzinganjira H, Kanyama C, Hosseinipour MC, Chawinga C, Meintjes G, Schutz C, Comins K, Singh A, Muzoora C, Jjunju S, Nuwagira E, Mosepele M, Leeme T, Siamisang K, Ndhlovu CE, Hlupeni A, Mutata C, van Widenfelt E, Chen T, Wang D, Hope W, Boyer-Chammard T, Loyse A, Molloy SF, Youssouf N, Lortholary O, Lalloo DG, Jaffar S, Harrison TS, Ambition Study Group . 2022. Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. N Engl J Med 386:1109–1120. doi:10.1056/NEJMoa2111904. - DOI - PMC - PubMed
-
- Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen M-H, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. doi:10.1086/649858. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
