The Phytophthora sojae nuclear effector PsAvh110 targets a host transcriptional complex to modulate plant immunity
- PMID: 36222564
- PMCID: PMC9806631
- DOI: 10.1093/plcell/koac300
The Phytophthora sojae nuclear effector PsAvh110 targets a host transcriptional complex to modulate plant immunity
Abstract
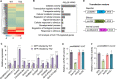
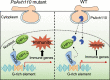
Plants have evolved sophisticated immune networks to restrict pathogen colonization. In response, pathogens deploy numerous virulent effectors to circumvent plant immune responses. However, the molecular mechanisms by which pathogen-derived effectors suppress plant defenses remain elusive. Here, we report that the nucleus-localized RxLR effector PsAvh110 from the pathogen Phytophthora sojae, causing soybean (Glycine max) stem and root rot, modulates the activity of a transcriptional complex to suppress plant immunity. Soybean like-heterochromatin protein 1-2 (GmLHP1-2) and plant homeodomain finger protein 6 (GmPHD6) form a transcriptional complex with transcriptional activity that positively regulates plant immunity against Phytophthora infection. To suppress plant immunity, the nuclear effector PsAvh110 disrupts the assembly of the GmLHP1-2/GmPHD6 complex via specifically binding to GmLHP1-2, thus blocking its transcriptional activity. We further show that PsAvh110 represses the expression of a subset of immune-associated genes, including BRI1-associated receptor kinase 1-3 (GmBAK1-3) and pathogenesis-related protein 1 (GmPR1), via G-rich elements in gene promoters. Importantly, PsAvh110 is a conserved effector in different Phytophthora species, suggesting that the PsAvh110 regulatory mechanism might be widely utilized in the genus to manipulate plant immunity. Thus, our study reveals a regulatory mechanism by which pathogen effectors target a transcriptional complex to reprogram transcription.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures






Similar articles
-
Homologous RXLR effectors from Hyaloperonospora arabidopsidis and Phytophthora sojae suppress immunity in distantly related plants.Plant J. 2012 Dec;72(6):882-93. doi: 10.1111/j.1365-313X.2012.05079.x. Epub 2012 Oct 26. Plant J. 2012. PMID: 22709376
-
GmBTB/POZ promotes the ubiquitination and degradation of LHP1 to regulate the response of soybean to Phytophthora sojae.Commun Biol. 2021 Mar 19;4(1):372. doi: 10.1038/s42003-021-01907-7. Commun Biol. 2021. PMID: 33742112 Free PMC article.
-
PcAvh87, a virulence essential RxLR effector of Phytophthora cinnamomi suppresses host defense and induces cell death in plant nucleus.Microbiol Res. 2024 Sep;286:127789. doi: 10.1016/j.micres.2024.127789. Epub 2024 Jun 10. Microbiol Res. 2024. PMID: 38870619
-
Effectors of Phytophthora pathogens are powerful weapons for manipulating host immunity.Planta. 2019 Aug;250(2):413-425. doi: 10.1007/s00425-019-03219-x. Epub 2019 Jun 26. Planta. 2019. PMID: 31243548 Review.
-
Phytophthora sojae effectors orchestrate warfare with host immunity.Curr Opin Microbiol. 2018 Dec;46:7-13. doi: 10.1016/j.mib.2018.01.008. Epub 2018 Feb 22. Curr Opin Microbiol. 2018. PMID: 29454192 Review.
Cited by
-
Plant PR1 rescues condensation of the plastid iron-sulfur protein by a fungal effector.Nat Plants. 2024 Nov;10(11):1775-1789. doi: 10.1038/s41477-024-01811-y. Epub 2024 Oct 4. Nat Plants. 2024. PMID: 39367256
-
Rice stripe virus nonstructural protein 3 suppresses plant defence responses mediated by the MEL-SHMT1 module.Mol Plant Pathol. 2023 Nov;24(11):1359-1369. doi: 10.1111/mpp.13373. Epub 2023 Jul 5. Mol Plant Pathol. 2023. PMID: 37404045 Free PMC article.
-
Genome-Wide Identification and Characterization of Effector Candidates with Conserved Motif in Falciphora oryzae.Int J Mol Sci. 2024 Jan 4;25(1):650. doi: 10.3390/ijms25010650. Int J Mol Sci. 2024. PMID: 38203820 Free PMC article.
-
The effectors of Phytophthora infestans impact host immunity upon regulation of antagonistic hormonal activities.Planta. 2023 Aug 2;258(3):59. doi: 10.1007/s00425-023-04215-y. Planta. 2023. PMID: 37530861
-
Phytophthora sojae Effector PsAvh113 Targets Transcription Factors in Nicotiana benthamiana.J Fungi (Basel). 2024 Apr 27;10(5):318. doi: 10.3390/jof10050318. J Fungi (Basel). 2024. PMID: 38786673 Free PMC article.
References
-
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 - PubMed
-
- Ayyanathan K, Lechner MS, Bell P, Maul GG, Schultz DC, Yamada Y, Tanaka K, Torigoe K, Rauscher FJ 3rd (2003) Regulated recruitment of HP1 to a Euchromatic gene induces mitotically heritable, epigenetic gene silencing: a mammalian cell culture model of gene variegation. Genes Dev 17: 1855–1869 - PMC - PubMed
-
- Beakes GW, Glockling SL, Sekimoto S (2012) The evolutionary phylogeny of the oomycete “fungi.” Protoplasma 249: 3–19 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

