Active tactile discrimination is coupled with and modulated by the cardiac cycle
- PMID: 36222653
- PMCID: PMC9671494
- DOI: 10.7554/eLife.78126
Active tactile discrimination is coupled with and modulated by the cardiac cycle
Abstract
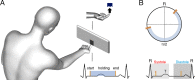
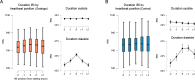
Perception and cognition are modulated by the phase of the cardiac signal in which the stimuli are presented. This has been shown by locking the presentation of stimuli to distinct cardiac phases. However, in everyday life sensory information is not presented in this passive and phase-locked manner, instead we actively move and control our sensors to perceive the world. Whether active sensing is coupled and modulated with the cardiac cycle remains largely unknown. Here, we recorded the electrocardiograms of human participants while they actively performed a tactile grating orientation task. We show that the duration of subjects' touch varied as a function of the cardiac phase in which they initiated it. Touches initiated in the systole phase were held for longer periods of time than touches initiated in the diastole phase. This effect was most pronounced when elongating the duration of the touches to sense the most difficult gratings. Conversely, while touches in the control condition were coupled to the cardiac cycle, their length did not vary as a function of the phase in which these were initiated. Our results reveal that we actively spend more time sensing during systole periods, the cardiac phase associated with lower perceptual sensitivity (vs. diastole). In line with interoceptive inference accounts, these results indicate that we actively adjust the acquisition of sense data to our internal bodily cycles.
Keywords: active inference; active sensing; cardiac cycle; human; interoception; neuroscience; predictive coding; tactile discrimination; touch.
Plain language summary
Most of what is known about human senses comes from experiments under laboratory conditions where participants stay still and stimuli are presented to them by the scientists. However, this approach does not reflect what happens in real life as we move around, changing the position of our eyes, heads and hands, to actively sense the world. Our perception also changes depending on what is going on inside our bodies and minds at any one time. For instance, our sensitivity to touch varies during the two phases of our heartbeat: people are less perceptive to being touched during systole (when the heart ejects blood), compared to when they are touched during diastole (when the heart refills with blood). But it was unclear if this relationship influences how we actively touch and sense objects. For instance, do people seek touch in a particular phase of their heartbeat, and how does this change their response to the object? To investigate, Galvez-Pol et al. traced people’s heartbeats while they actively touched different objects. Without looking, the participants had to work out whether the objects had vertical or horizontal grooves. Although they did not start their touches in a specific phase of the heartbeat, their hearts did influence their behaviour. If they started the touch during systole, they held their fingers over the object for longer. The effect was especially noticeable when it was difficult to discriminate the objects’ grooves. Galvez-Pol et al. reasoned that this was down to participants having to compensate for the loss in touch sensitivity during the systole phase of their heartbeat. This suggests that people actively adjust how they acquire sensory information, such as touch, based on how their bodily functions alter their senses. These findings provide a starting point for future studies investigating how internal bodily fluctuations impact how we sense and respond to things in real world scenarios. This could potentially shed light on the differences between the way neurotypical and neurodivergent individuals sense the world.
© 2022, Galvez-Pol et al.
Conflict of interest statement
AG, PV, JV, JK No competing interests declared
Figures



Comment in
-
In sync with the heart.Elife. 2022 Nov 17;11:e84298. doi: 10.7554/eLife.84298. Elife. 2022. PMID: 36394452 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

