Therapy-Induced Senescence Enhances the Efficacy of HER2-Targeted Antibody-Drug Conjugates in Breast Cancer
- PMID: 36222720
- PMCID: PMC9755966
- DOI: 10.1158/0008-5472.CAN-22-0787
Therapy-Induced Senescence Enhances the Efficacy of HER2-Targeted Antibody-Drug Conjugates in Breast Cancer
Abstract
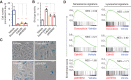
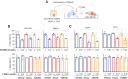
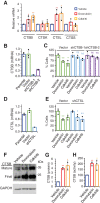
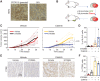
Antibody-drug conjugates (ADC) are antineoplastic agents recently introduced into the antitumor arsenal. T-DM1, a trastuzumab-based ADC that relies on lysosomal processing to release the payload, is approved for HER2-positive breast cancer. Next-generation ADCs targeting HER2, such as [vic-]trastuzumab duocarmazine (SYD985), bear linkers cleavable by lysosomal proteases and membrane-permeable drugs, mediating a bystander effect by which neighboring antigen-negative cells are eliminated. Many antitumor therapies, like DNA-damaging agents or CDK4/6 inhibitors, can induce senescence, a cellular state characterized by stable cell-cycle arrest. Another hallmark of cellular senescence is the enlargement of the lysosomal compartment. Given the relevance of the lysosome to the mechanism of action of ADCs, we hypothesized that therapies that induce senescence would potentiate the efficacy of HER2-targeting ADCs. Treatment with the DNA-damaging agent doxorubicin and CDK4/6 inhibitor induced lysosomal enlargement and senescence in several breast cancer cell lines. While senescence-inducing drugs did not increase the cytotoxic effect of ADCs on target cells, the bystander effect was enhanced when HER2-negative cells were cocultured with HER2-low cells. Knockdown experiments demonstrated the importance of cathepsin B in the enhanced bystander effect, suggesting that cathepsin B mediates linker cleavage. In breast cancer patient-derived xenografts, a combination treatment of CDK4/6 inhibitor and SYD985 showed improved antitumor effects over either treatment alone. These data support the strategy of combining next-generation ADCs targeting HER2 with senescence-inducing therapies for tumors with heterogenous and low HER2 expression.
Significance: Combining ADCs against HER2-positive breast cancers with therapies that induce cellular senescence may improve their therapeutic efficacy by facilitating a bystander effect against antigen-negative tumor cells.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Pegram MD, Miles D, Tsui CK, Zong Y. HER2-Overexpressing/amplified breast cancer as a testing ground for antibody-drug conjugate drug development in solid tumors. Clin Cancer Res 2019;26:775–86. - PubMed
-
- Cortés J, Kim S-B, Chung W-P, Im S-A, Park YH, Hegg R, et al. . LBA1 trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): results of the randomized phase III DESTINY-Breast03 study. Ann Oncol 2021;32:S1287–8.
-
- Phillips GDL, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. . Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008;68:9280–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

