Matching-Adjusted Indirect Comparison of Long-Term Efficacy and Safety Outcomes for Calcipotriol Plus Betamethasone Dipropionate Foam Versus Halobetasol Proprionate Plus Tazarotene Lotion in the Treatment of Plaque Psoriasis
- PMID: 36223060
- PMCID: PMC9588120
- DOI: 10.1007/s13555-022-00824-9
Matching-Adjusted Indirect Comparison of Long-Term Efficacy and Safety Outcomes for Calcipotriol Plus Betamethasone Dipropionate Foam Versus Halobetasol Proprionate Plus Tazarotene Lotion in the Treatment of Plaque Psoriasis
Abstract
Introduction: To date, there have been no head-to-head clinical studies comparing calcipotriol 0.005% plus betamethasone dipropionate 0.064% (Cal/BD) aerosol foam and halobetasol propionate 0.01% plus tazarotene 0.045% (HP/Taz) lotion for the treatment of plaque psoriasis. However, the efficacy of 4 weeks of Cal/BD foam and 8 weeks of HP/Taz lotion has been compared using a matching-adjusted indirect comparison (MAIC) approach. Here, we compare the efficacy and safety of Cal/BD foam and HP/Taz lotion for up to 52 weeks.
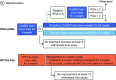
Methods: An unanchored MAIC was conducted using individual patient data from the PSO-LONG Cal/BD foam trial and a 52-week, open-label phase 3 study of HP/Taz lotion (NCT02462083). Key outcomes of interest were Physician's Global Assessment (PGA) success (PGA 0/1 with ≥ 2-point improvement) after 4 or 8 weeks of open-label therapy; the proportion of patients who had body surface area affected (BSA) ≤ 3 after open-label therapy who maintained BSA ≤ 3 to week 52; and adverse events (AEs).
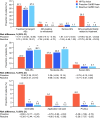
Results: After matching, patients were statistically significantly more likely to have PGA success after 4 weeks of Cal/BD foam than after 8 weeks of HP/Taz lotion (84.5% versus 54.4%; p < 0.01). At week 52, 92.5% and 92.4% of patients receiving proactive and reactive Cal/BD foam, respectively, maintained BSA ≤ 3, compared with 49.3% of those treated with HP/Taz lotion (both p < 0.01). Treatment-related AEs, AEs leading to withdrawal, and AEs associated with drug application (dermatitis, application site pain, and pruritus) were significantly rarer with Cal/BD foam than with HP/Taz lotion (all p < 0.01).
Conclusions: Cal/BD aerosol foam demonstrated significantly greater efficacy than HP/Taz lotion, and had a more favorable safety profile, compared with HP/Taz lotion, for up to 52 weeks. Proactive Cal/BD foam maintenance therapy and reactive use of Cal/BD foam following relapse both had significant advantages over HP/Taz lotion.
Keywords: Betamethasone dipropionate; Calcipotriol; Foam; Halobetasol propionate; Psoriasis; Tazarotene.
© 2022. The Author(s).
Figures

Similar articles
-
Effectiveness comparison and incremental cost-per-responder analysis of calcipotriene 0.005%/betamethasone dipropionate 0.064% foam vs. halobetasol 0.01%/tazarotene 0.045% lotion for plaque psoriasis: a matching-adjusted indirect comparative analysis.J Med Econ. 2020 Jun;23(6):641-649. doi: 10.1080/13696998.2020.1722139. Epub 2020 Mar 2. J Med Econ. 2020. PMID: 31985301
-
Matching-adjusted indirect comparison of efficacy outcomes in trials of calcipotriol plus betamethasone dipropionate foam and cream formulations for the treatment of plaque psoriasis.J Dermatolog Treat. 2022 Nov;33(7):3005-3013. doi: 10.1080/09546634.2022.2095330. Epub 2022 Jul 25. J Dermatolog Treat. 2022. PMID: 35875991
-
Efficacy, Safety, and Tolerability of a Halobetasol 0.01% /Tazarotene 0.045% Fixed Combination in the Treatment of Severe Localized Plaque Psoriasis: Post Hoc Analysis of Two Phase III Randomized Controlled Trials.J Drugs Dermatol. 2019 Oct 1;18(10):1012-1018. J Drugs Dermatol. 2019. PMID: 31584780 Clinical Trial.
-
Treating Psoriasis With Halobetasol Propionate and Tazarotene Combination: A Review of Phase II and III Clinical Trials.Ann Pharmacother. 2020 Sep;54(9):872-878. doi: 10.1177/1060028020910439. Epub 2020 Mar 4. Ann Pharmacother. 2020. PMID: 32126800
-
Fixed-Combination Calcipotriene Plus Betamethasone Dipropionate Aerosol Foam Is Well Tolerated in Patients with Psoriasis Vulgaris: Pooled Data from Three Randomized Controlled Studies.Skinmed. 2017 Apr 1;15(2):119-124. eCollection 2017. Skinmed. 2017. PMID: 28528605 Review.
Cited by
-
Cost-effectiveness of edaravone dexborneol versus dl-3-n-butylphthalide for the treatment of acute ischemic stroke: a Chinese health care perspective.BMC Public Health. 2024 Feb 12;24(1):436. doi: 10.1186/s12889-024-17959-3. BMC Public Health. 2024. PMID: 38347500 Free PMC article.
-
Topical proactive therapy in dermatology. A scoping review.Postepy Dermatol Alergol. 2023 Aug;40(4):510-517. doi: 10.5114/ada.2023.129454. Epub 2023 Sep 1. Postepy Dermatol Alergol. 2023. PMID: 37692271 Free PMC article.
References
-
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. - DOI - PubMed
-
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(871–81):e1–30. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

