Longer-Term All-Cause and Cardiovascular Mortality With Intensive Blood Pressure Control: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 36223105
- PMCID: PMC9558058
- DOI: 10.1001/jamacardio.2022.3345
Longer-Term All-Cause and Cardiovascular Mortality With Intensive Blood Pressure Control: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: The Systolic Blood Pressure Intervention Trial (SPRINT) showed that intensive blood pressure control reduced cardiovascular morbidity and mortality. However, the legacy effect of intensive treatment is unknown.
Objective: To evaluate the long-term effects of randomization to intensive treatment with the incidence of cardiovascular and all-cause mortality approximately 4.5 years after the trial ended.
Design, setting, and participants: In this secondary analysis of a multicenter randomized clinical trial, randomization began on November 8, 2010, the trial intervention ended on August 20, 2015, and trial close-out visits occurred through July 2016. Patients 50 years and older with hypertension and increased cardiovascular risk but without diabetes or history of stroke were included from 102 clinic sites in the US and Puerto Rico. Analyses were conducted between October 2021 and February 2022.
Interventions: Randomization to systolic blood pressure (SBP) goal of less than 120 mm Hg (intensive treatment group; n = 4678) vs less than 140 mm Hg (standard treatment group; n = 4683).
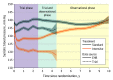
Main outcomes and measures: Extended observational follow-up for mortality via the US National Death Index from 2016 through 2020. In a subset of 2944 trial participants, outpatient SBP from electronic health records during and after the trial were examined.
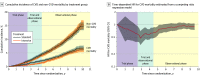
Results: Among 9361 randomized participants, the mean (SD) age was 67.9 (9.4) years, and 3332 (35.6%) were women. Over a median (IQR) intervention period of 3.3 (2.9-3.9) years, intensive treatment was beneficial for both cardiovascular mortality (hazard ratio [HR], 0.66; 95% CI, 0.49-0.89) and all-cause mortality (HR, 0.83; 95% CI, 0.68-1.01). However, at the median (IQR) total follow-up of 8.8 (8.3-9.3) years, there was no longer evidence of benefit for cardiovascular mortality (HR, 1.02; 95% CI, 0.84-1.24) or all-cause mortality (HR, 1.08; 95% CI, 0.94-1.23). In a subgroup of participants, the estimated mean outpatient SBP among participants randomized to intensive treatment increased from 132.8 mm Hg (95% CI, 132.0-133.7) at 5 years to 140.4 mm Hg (95% CI, 137.8-143.0) at 10 years following randomization.
Conclusions and relevance: The beneficial effect of intensive treatment on cardiovascular and all-cause mortality did not persist after the trial. Given increasing outpatient SBP levels in participants randomized to intensive treatment following the trial, these results highlight the importance of consistent long-term management of hypertension.
Trial registration: ClinicalTrials.gov Identifier: NCT01206062.
Conflict of interest statement
Figures


Comment in
-
Blood Pressure Control After SPRINT-Back to Reality.JAMA Cardiol. 2022 Nov 1;7(11):1146-1147. doi: 10.1001/jamacardio.2022.3357. JAMA Cardiol. 2022. PMID: 36223086 No abstract available.
References
-
- Roth GA, Mensah GA, Johnson CO, et al. ; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group . Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021. doi:10.1016/j.jacc.2020.11.010 - DOI - PMC - PubMed
-
- Rahimi K, Bidel Z, Nazarzadeh M, et al. ; Blood Pressure Lowering Treatment Trialists’ Collaboration . Age-stratified and blood-pressure-stratified effects of blood-pressure-lowering pharmacotherapy for the prevention of cardiovascular disease and death: an individual participant-level data meta-analysis. Lancet. 2021;398(10305):1053-1064. doi:10.1016/S0140-6736(21)01921-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 HL136679/HL/NHLBI NIH HHS/United States
- R01 AG055606/AG/NIA NIH HHS/United States
- P30 GM103337/GM/NIGMS NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- R01 AG065805/AG/NIA NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

