Sensitivity and Diagnostic Yield of the First SARS-CoV-2 Nucleic Acid Amplification Test Performed for Patients Presenting to the Hospital
- PMID: 36223119
- PMCID: PMC9557877
- DOI: 10.1001/jamanetworkopen.2022.36288
Sensitivity and Diagnostic Yield of the First SARS-CoV-2 Nucleic Acid Amplification Test Performed for Patients Presenting to the Hospital
Abstract
Importance: Early and accurate diagnostic testing for SARS-CoV-2 is essential to initiate appropriate treatment and infection control and prevention measures among patients presenting to the hospital.
Objective: To evaluate the diagnostic sensitivity of the SARS-CoV-2 nucleic acid amplification test (NAAT) performed within 24 hours of arrival to the emergency department among a nationally representative sample of patients.
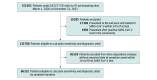
Design, setting, and participants: This diagnostic study was conducted at 47 hospitals across 7 provinces in Canada participating in the Canadian COVID-19 Rapid Response Emergency Department Network among consecutive eligible patients presenting to a participating emergency department who were tested for SARS-CoV-2 from March 1, 2020, to December 31, 2021. Patients not tested within 24 hours of arrival and those presenting with a positive result from a test performed in the community were excluded.
Main outcomes and measures: The primary outcome was a positive result from the SARS-CoV-2 NAAT. Outcome measures were the diagnostic sensitivity and yield of the SARS-CoV-2 NAAT.
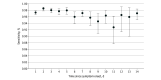
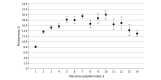
Results: Of 132 760 eligible patients (66 433 women [50.0%]; median age, 57 years [IQR, 37-74 years]), 17 174 (12.9%) tested positive for SARS-CoV-2 within 14 days of their first NAAT. The diagnostic sensitivity of the SARS-CoV-2 NAAT was 96.2% (17 070 of 17 740 [95% CI, 95.9%-96.4%]) among all of the tests performed. Estimates ranged from a high of 97.7% (1710 of 1751 [95% CI, 96.8%-98.3%]) on day 2 of symptoms to a low of 90.4% (170 of 188 [95% CI, 85.3%-94.2%]) on day 11 of symptoms among patients presenting with COVID-19 symptoms. Among patients reporting COVID-19 symptoms, the sensitivity of the SARS-CoV-2 NAAT was 97.1% (11 870 of 12 225 [95% CI, 96.7%-97.3%]) compared with 87.6% (812 of 927 [95% CI, 85.2%-89.6%]) among patients without COVID-19 symptoms. The diagnostic yield of the SARS-CoV-2 NAAT was 12.0% (18 985 of 158 004 [95% CI, 11.8%-12.2%]) and varied from a high of 20.0% (445 of 2229 [95% CI, 18.3%-21.6%]) among patients tested on day 10 after symptom onset to a low of 8.1% (1686 of 20 719 [95% CI, 7.7%-8.5%]) among patients presenting within the first 24 hours of symptom onset.
Conclusions and relevance: This study suggests that the diagnostic sensitivity was high for the first SARS-CoV-2 NAAT performed in the hospital and did not vary significantly by symptom duration. Repeated testing of patients with negative test results should be avoided unless their pretest probability of disease is high.
Conflict of interest statement
Figures
Similar articles
-
Association of SARS-CoV-2 Seropositive Antibody Test With Risk of Future Infection.JAMA Intern Med. 2021 May 1;181(5):672-679. doi: 10.1001/jamainternmed.2021.0366. JAMA Intern Med. 2021. PMID: 33625463 Free PMC article.
-
Viral load may impact the diagnostic performance of nasal swabs in nucleic acid amplification test and quantitative antigen test for SARS-CoV-2 detection.J Infect Chemother. 2022 Nov;28(11):1590-1593. doi: 10.1016/j.jiac.2022.07.023. Epub 2022 Aug 8. J Infect Chemother. 2022. PMID: 35953013 Free PMC article.
-
Diagnostic yield of screening for SARS-CoV-2 among patients admitted to hospital for alternate diagnoses: an observational cohort study.BMJ Open. 2022 Aug 10;12(8):e057852. doi: 10.1136/bmjopen-2021-057852. BMJ Open. 2022. PMID: 35948378 Free PMC article.
-
Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2: A Systematic Review and Meta-analysis.JAMA Intern Med. 2021 Mar 1;181(3):353-360. doi: 10.1001/jamainternmed.2020.8876. JAMA Intern Med. 2021. PMID: 33449069 Free PMC article.
-
Strategies That Facilitate Extraction-Free SARS-CoV-2 Nucleic Acid Amplification Tests.Viruses. 2022 Jun 15;14(6):1311. doi: 10.3390/v14061311. Viruses. 2022. PMID: 35746782 Free PMC article. Review.
Cited by
-
Comparing methods to classify admitted patients with SARS-CoV-2 as admitted for COVID-19 versus with incidental SARS-CoV-2: A cohort study.PLoS One. 2023 Sep 26;18(9):e0291580. doi: 10.1371/journal.pone.0291580. eCollection 2023. PLoS One. 2023. PMID: 37751455 Free PMC article.
-
Accuracy of the Canadian COVID-19 Mortality Score (CCMS) to predict in-hospital mortality among vaccinated and unvaccinated patients infected with Omicron: a cohort study.BMJ Open. 2024 Nov 19;14(11):e083280. doi: 10.1136/bmjopen-2023-083280. BMJ Open. 2024. PMID: 39566942 Free PMC article.
-
Heatstroke presentations to urban hospitals during BC's extreme heat event: lessons for the future.CJEM. 2024 Feb;26(2):111-118. doi: 10.1007/s43678-023-00622-y. Epub 2023 Dec 28. CJEM. 2024. PMID: 38153655 Free PMC article. Review.
-
Administrative data ICD-10 diagnostic codes identifies most lab-confirmed SARS-CoV-2 admissions but misses many discharged from the Emergency Department.Sci Rep. 2024 Mar 12;14(1):6008. doi: 10.1038/s41598-023-49501-7. Sci Rep. 2024. PMID: 38472258 Free PMC article.
-
Post-COVID-19 condition symptoms among emergency department patients tested for SARS-CoV-2 infection.Nat Commun. 2024 Sep 30;15(1):8449. doi: 10.1038/s41467-024-52404-4. Nat Commun. 2024. PMID: 39349926 Free PMC article.
References
-
- Infectious Diseases Society of America . IDSA guidelines on the diagnosis of COVID-19: molecular diagnostic testing. Updated December 23, 2020. Accessed March 10, 2022. https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnost...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

